HPCP resin with polyhydroxy structure and preparation method thereof
A technology of cyclotriphosphazene and polyhydroxyl, which is applied in the field of cyclotriphosphazene resin and its preparation, and can solve the problems of high price, lack of large-scale application, difficult processing and moldability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Synthesis of Ethylene Glycol Etherified Dihydroxycyclotriphosphazene Resin
[0057] 1) Product Description
[0058] Product Name: Ethylene Glycol Etherified Dihydroxycyclotriphosphazene Resin
[0059] Molecular weight: 381.14
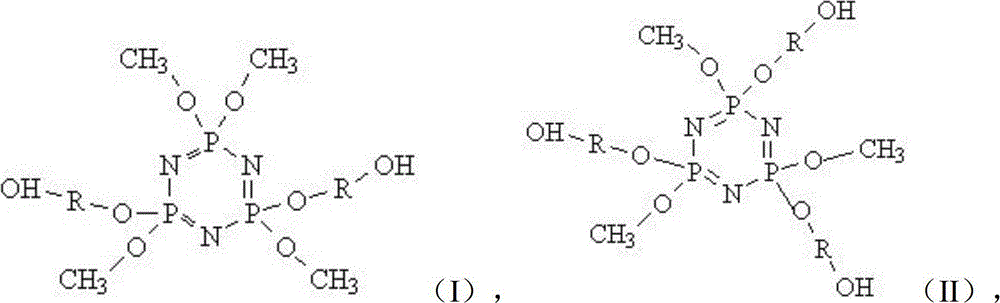
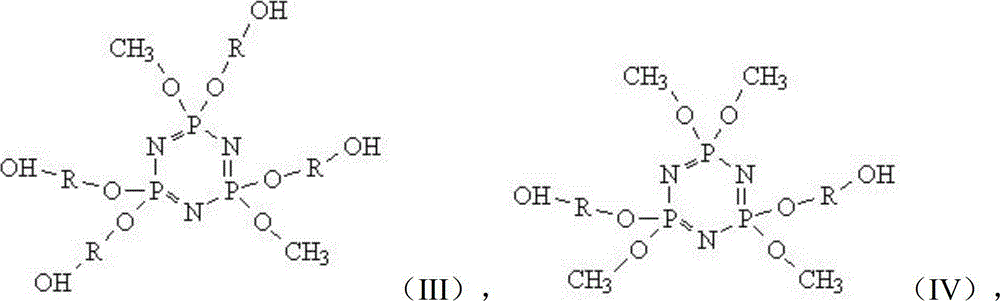
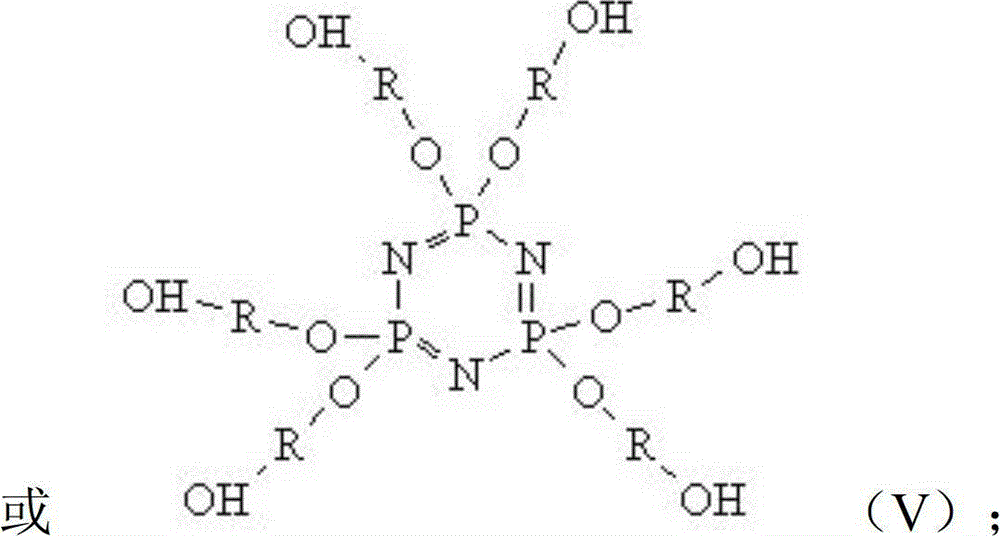
[0060] Molecular Structure:
[0061]
[0062] Quality Index:
[0063] Appearance: light yellow transparent viscous liquid
[0064] Acid value: (mgKOH / g) ≤0.2
[0065] Chromaticity: (APHA) ≤55
[0066] Ignition point: ℃ ------
[0067] Specific gravity: (20 / 25°C) 1.117-1.123
[0068] Moisture: Wt.% ≤0.5
[0069] Soluble in water, alcohol, phosphate ester and other solvents.
[0070] Uses: Reactive P-N polyurethane resin flame retardant. Flame retardant polyether starter.
[0071] 2) Synthesis technology
[0072] The hexamethoxycyclotriphosphazene resin and ethylene glycol undergo ether exchange reaction to generate ethylene glycol etherified dihydroxycyclotriphosphazene resin.
[0073] Raw material ratio
[0074]
[0075] Che...
Embodiment 2
[0079] Ethylene Glycol Etherified Trihydroxycyclotriphosphoryl Resin
[0080] Raw material ratio
[0081]
[0082] Chemical reaction formula:
[0083]
[0084] In the reactor, add ethylene glycol: 37.24Kg, add hexamethoxycyclotriphosphazene resin (VI): 64.23Kg, add zirconium sulfate catalyst: 1.2Kg. Raise the temperature to 110-130°C, carry out the ether exchange reaction for 4-5 hours, distill off the methanol generated in the reaction, cool to room temperature, filter out the catalyst (reuse 8-10 times), and obtain the product: Ethylene glycol etherified trihydroxyl ring Triphosphonitrile resin: 81.25Kg. Yield: 98.8%, viscosity: 7680mPa.S / 25℃.
Embodiment 3
[0086] Ethylene Glycol Etherified Tetrahydroxycyclotriphosphoryl Resin
[0087] Raw material ratio
[0088]
[0089] Chemical reaction formula:
[0090]
[0091] In the reaction kettle, add ethylene glycol: 49.66Kg, add hexamethoxy cyclotriphosphazene resin (VI): 64.23Kg, add zirconium sulfate: 1.3Kg. Raise the temperature to 110-130°C, carry out the ether exchange reaction for 4-5 hours, distill off the methanol generated in the reaction, cool to room temperature, filter out the catalyst (reuse 8-10 times), and obtain the product: tetrahydroxyl ring etherified with ethylene glycol Triphosphazene resin: 87.26Kg. Yield: 98.9%, viscosity: 7460mPa.S / 25℃.
PUM
| Property | Measurement | Unit |
|---|---|---|
| oxygen index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com