Cell separation medium and cell separation method
A technology for separating medium and nuclear cells, which is applied in the field of compound cell separation medium and cell separation, can solve the problems of increasing cost, prolonging the production cycle, increasing the complexity of industrialized cell production, etc., and achieving low raw material uncertainty and high safety Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1, the preparation of formula A separation medium
[0062] In an environment with local 100-level laminar flow purification conditions, at 20°C, those skilled in the art who have been trained in aseptic techniques used conventional methods to respectively prepare Dextran 70, hydroxyethyl Starch (200 / 0.5) and a certain amount of sodium diatrizoate are fully dissolved in sterile water for injection to form a clear and transparent colorless or slightly yellow aqueous solution. The mass fractions of the three substances are 2.25%, 2.25%, and 9.0%, respectively. Then filter through a 0.22μm filter membrane into a sterile, endotoxin-free container.
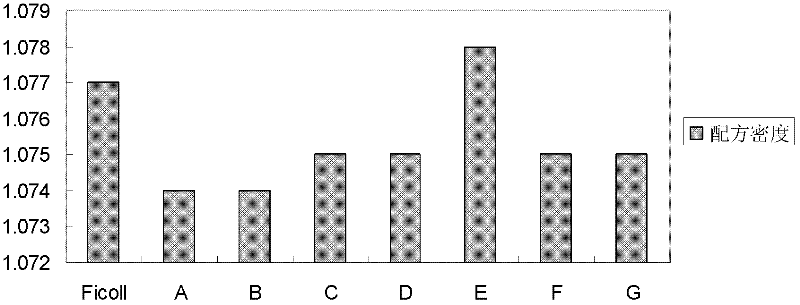
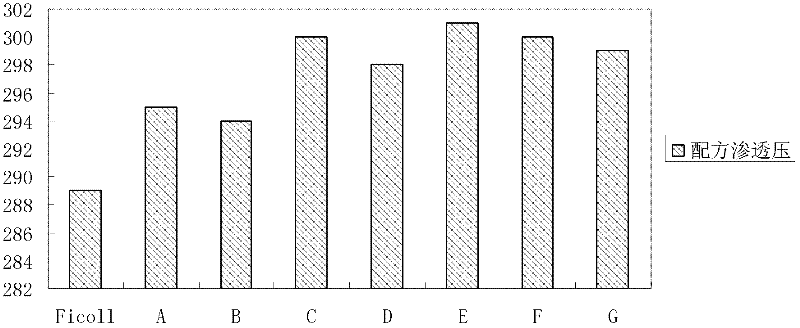
[0063] At 20°C, the main characteristic parameters of the mixed aqueous solution include: (1) The density is 1.074g / cm 3 (See figure 1 , refer to method ); (2) osmotic pressure is physiological osmotic pressure (see figure 2 , between 285mOsm / kg and 310mOsm / kg) (refer to method ); (3) pH is between 6.0 and 9.0; (4)...
Embodiment 2
[0073] Embodiment 2, the preparation of formula B separation medium
[0074] In an environment with local 100-level laminar flow purification conditions, at 20°C, those skilled in the art who have been trained in aseptic techniques used conventional methods to respectively prepare Dextran 70, hydroxyl Ethyl starch (200 / 0.5) and a certain amount of sodium diatrizoate are fully dissolved in sterile water for injection to form a clear and transparent colorless or slightly yellow aqueous solution. The mass fractions of the three substances are 3.0%, 1.5%, and 9.0%, respectively. %, and then filtered through a 0.22μm filter membrane into a sterile, endotoxin-free container.
[0075] At 20°C, the main characteristic parameters of the mixed aqueous solution include: (1) The density is 1.074g / cm 3 (See figure 1 , refer to method ); (2) osmotic pressure is physiological osmotic pressure (see figure 2 , between 285mOsm / kg and 310mOsm / kg) (refer to method ); (3) pH between 6.0 and 9....
Embodiment 3
[0077] Embodiment 3, the preparation of formula C separation medium
[0078] In an environment with local 100-level laminar flow purification conditions, at 20°C, those skilled in the art who have been trained in aseptic techniques used conventional methods to respectively prepare Dextran 70, hydroxyl Ethyl starch (200 / 0.5) and a certain amount of sodium diatrizoate are fully dissolved in sterilized water for injection to form a clear and transparent colorless or slightly yellow product with mass fractions of 2.45%, 2.45%, and 9.0% respectively. The aqueous solution is then filtered through a 0.22μm filter membrane into a sterile, endotoxin-free container.
[0079] At 20°C, the main characteristic parameters of the mixed aqueous solution include: (1) The density is 1.075g / cm 3 (See figure 1 , refer to method ); (2) osmotic pressure is physiological osmotic pressure (see figure 2 , between 285mOsm / kg and 310mOsm / kg) (refer to method ); (3) pH between 6.0 and 9.0 (see imag...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com