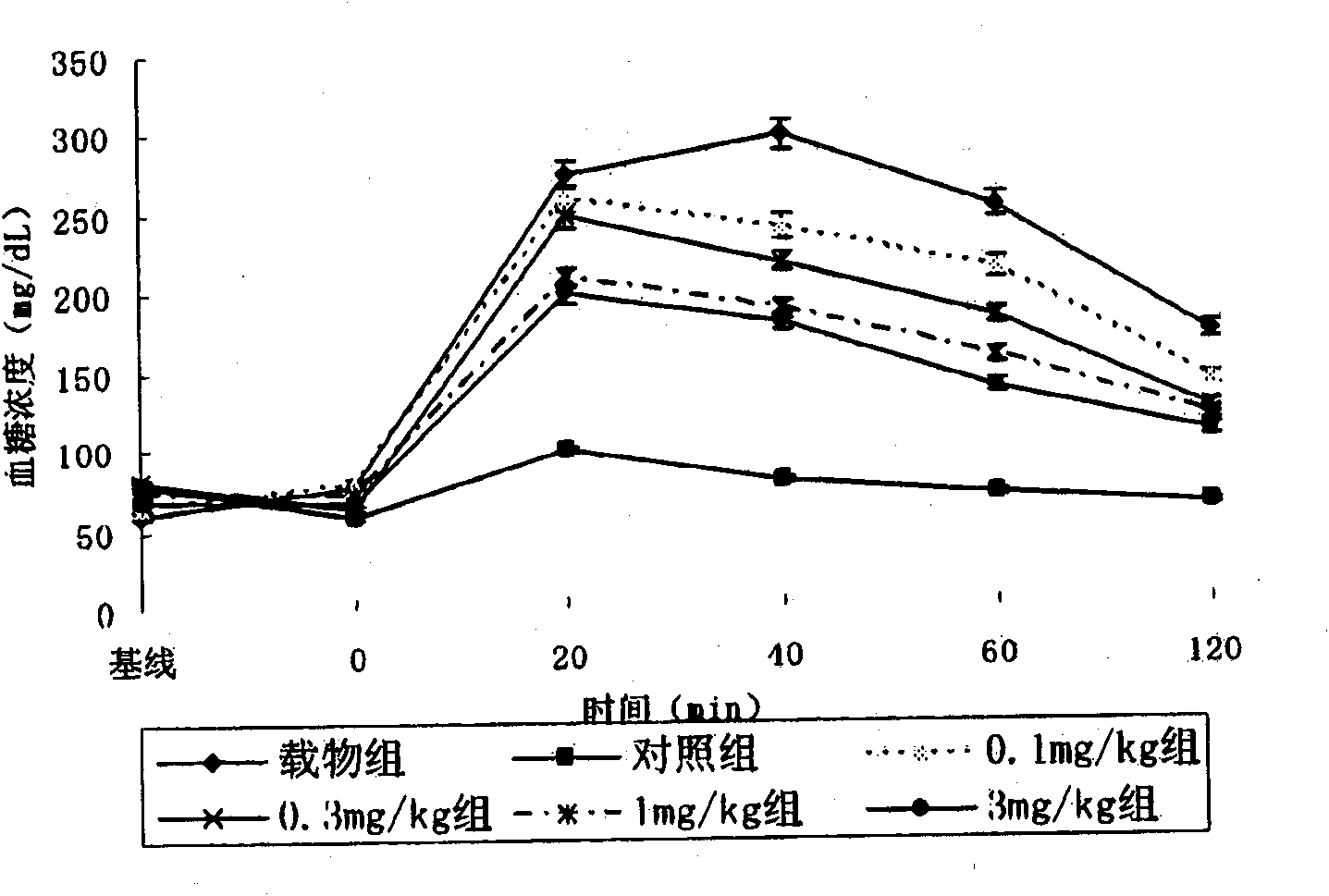
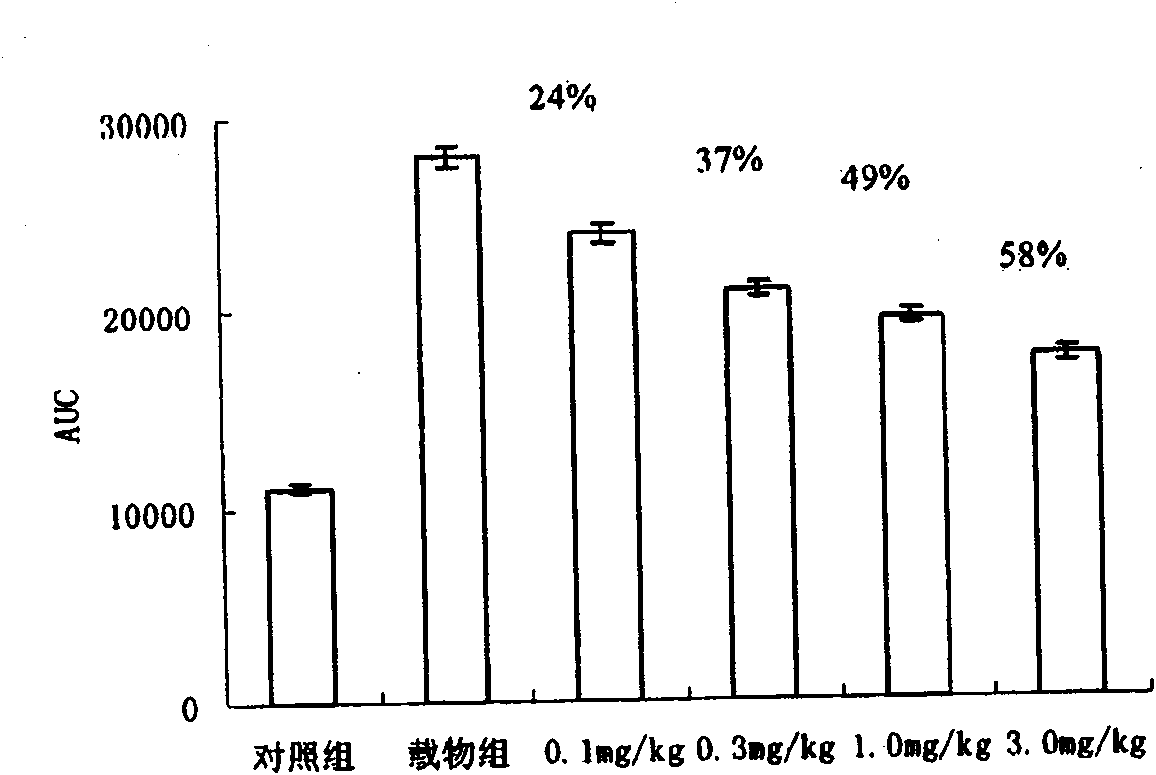
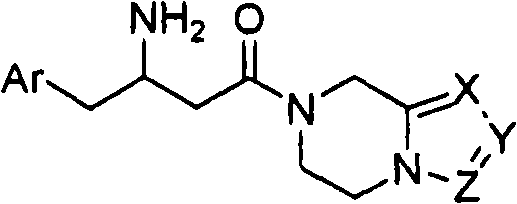
Beta-amino tetrahydropyrazine, tetrahydropyrimidine and tetrahydropyridine used as dipeptidy peptidase inhibitors for curing or preventing diabetes
An alkyl and phenyl technology, applied in the direction of urinary system diseases, diseases, metabolic diseases, etc., can solve problems that have not yet been studied, and there is no extensive research on DP-IV inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0257]
[0258] (R)-3-Amino-4-(2,5-difluorophenyl)-1-(5,6-dihydroimidazo[1,5-a]pyrazin-7(8H)-yl)butyl Alkan-1-one dihydrochloride
[0259] Step A. (R)-1-(2,5-difluorophenyl)-4-(5,6-dihydroimidazo[1,5-a]pyrazin-7(8H)-yl)-4 -Oxobutan-2-ylcarbamate tert-butyl ester
[0260] At 0°C, 5,6,7,8-tetrahydroimidazo[1,5-a]pyrazine (50.0mg, 0.40mmol) and Boc-(R)-3-amino-4-(2, To a solution of 5-difluorophenyl)butanoic acid (128.0 mg, 0.40 mmol) in dichloromethane (5 mL) was added HOBT (54.5 mg, 0.42 mmol). The reaction was stirred at 0 °C for 10 min, then EDC (96.6 mg, 0.50 mmol) was added. After removing the ice bath, the reaction was stirred at ambient temperature for 14 h. The mixture was concentrated and purified by HPLC (Gilson; YMC-Pack Pro C18 column, 100x20mm I.D.; solvent gradient from 10% acetonitrile, 90% water, and 0.1% trifluoroacetic acid to 90% acetonitrile, 10% water, and 0.1% trifluoroacetic acid) , to obtain 103 mg of the title compound as a foamy solid.
[0261]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com