Photo-curing monomer with ortho-phenolic hydroxyl structure, preparation method and bond thereof
A technology of o-phenolic hydroxyl and light curing, which is applied in the direction of non-macromolecular organic compound adhesives, cyanide reaction preparation, chemical instruments and methods, etc., to achieve the effects of low preparation cost, wide practicability, and easy operation
Inactive Publication Date: 2010-09-29
BEIJING UNIV OF CHEM TECH
View PDF3 Cites 42 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The main purpose of the present invention is to solve the common problem that the currently commercialized adhesives cannot have fast and long-lasting adhesion in both dry and wet states, and obtain fast and long-lasting adhesion that can bond both in dry and wet states. agent
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
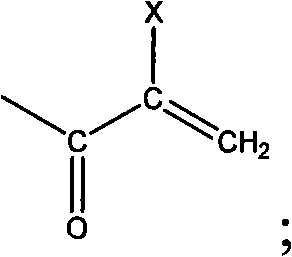
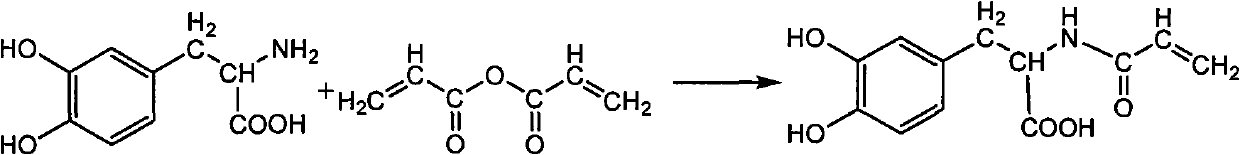
The invention discloses a photo-curing monomer with ortho-phenolic hydroxyl strucutre, a preparation method and bond thereof. In the formula I monomer, the compound which has crylic acid at two ends or crylic acid double bond at one end and methylacrylic acid double bond at the other end is added to the amidogen of dopamine and derivant thereof through a Michael addition method to form corresponding secondary amine or tertiary amine. In the formula II monomer, the double bond is introduced by (methyl) acrylic anhydride, (methyl) crylic acid and (methyl) acryloyl chloride. The photopolymerization bond comprises the following components by weight percent: 20 to 99.9 percent of the compound with the ortho-phenolic hydroxyl structure, 0 to 70 percent of activated thinner and / or solvent, 0.1 to 10 percent of evocating agent and 0 to 60 percent of natural macromolecule or modified outcome thereof. The bond can be bonded in dry state and wet state, is a quick and long lasting bond, realizes the organic bond of biological bond and photopolymerization, has the advantages of low manufacture cost, simple and convenient operation and high productivity and has extensive application foreground in the biological bond field.
Description
technical field The invention relates to a photocurable adhesive monomer, its preparation and its application as an adhesive, belonging to the field of adhesives. Background technique The o-phenolic hydroxyl group of dopamine or its derivatives can form a complex with metal ions in the body. If the o-phenolic hydroxyl group of dopamine or its derivatives is oxidized, or encounters oxidative substances such as tyrosinase in the body, o-quinone is formed The structure of the protein is a substance that can form a covalent cross-linked substance with the protein in the body. This property endows the compound with ortho-phenolic hydroxyl structure with good adhesion in both dry and wet environments. Photopolymerization (also known as photocuring) technology is a new green technology that came out in the 1960s. Photocuring technology: high efficiency, wide adaptability, economy, energy saving and environmental friendliness. These characteristics endow photopolymerization techn...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07C229/14C07C229/36C07C227/08C07C233/20C07C233/49C07C231/02C09J4/06
Inventor 杨冬芝牛睿聂俊
Owner BEIJING UNIV OF CHEM TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com