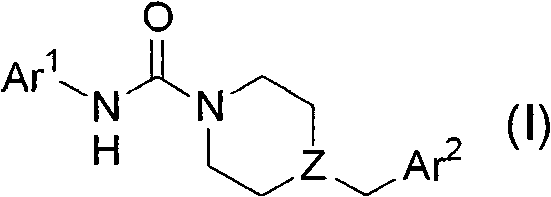
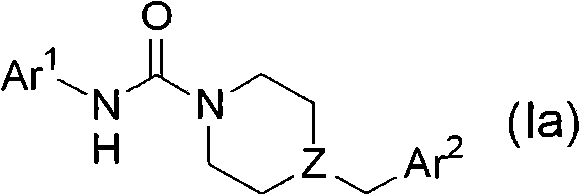
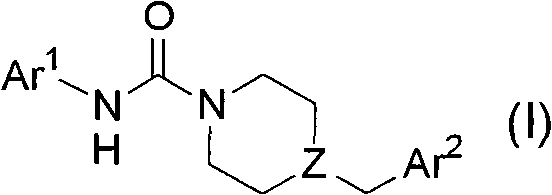
Heteroaryl-substituted urea modulators of fatty acid amide hydrolase
An alkyl and phenyl technology, applied in the field of heteroaryl-substituted urea regulators of fatty acid amide hydrolase, can solve the unknown problems of human osteoporosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0149] Example 1: 4-(2,2-Difluoro-benzo[1,3]dioxol-5-ylmethyl)-piperazine-1-carboxylic acid Benzo[d]iso evil Azol-3-ylamide trifluoroacetate
[0150]
[0151] Step A: Benzo[d]iso evil Azol-3-yl-phenylcarbamate Benzo[d]isoxazol-3-ylamide (3.0g) and ClCO 2 Ph (0.94mL) in dry CH 3 The mixture in CN (30 mL) was stirred at 70 °C for 23 hours. The reaction mixture was poured into deionized water, stirred for 30 minutes and filtered. The isolated solid was rinsed thoroughly with water and dried under high vacuum to afford 1.90 g (100%) of the title compound. MS: 255.1.
[0152] Step B: 1-(2,2-Difluoro-benzo[1,3]dioxol-5-ylmethyl)-piperazine Piperazine-1-carboxylate tert-butyl ester (20.0 g) and 2,2-difluoro-benzo[1,3]dioxole-5-carbaldehyde (14.8 mL) in DCE (208 mL) A 0°C solution of NaB(OAc) 3 H (31.8g) treatment. The mixture was allowed to warm to room temperature and stirred for 16 hours. The resulting mixture was cooled in an ice bath and treated with 10% aqu...
example 2
[0155] Example 2: 4-(2,2-Difluoro-benzo[1,3]dioxol-5-ylmethyl)-piperazine-1-carboxylic acid (3-Phenyl-[1,2,4]thiadiazol-5-yl)-amide trifluoroacetate
[0156]
[0157] MS: 460.5. 1 H NMR (CDCl 3 ): 10.66 (br s, 1H), 8.10-8.08 (m, 2H), 7.46-7.43 (m, 3H), 7.00 (s, 1H), 6.96 (d, J=8.4, 1H), 6.91-6.89 ( dd, J = 1.2, 7.8, 1H), 3.33 (br s, 6H), 2.15 (br s, 4H).
example 3
[0158] Example 3: 4-(2,2-Difluoro-benzo[1,3]dioxol-5-ylmethyl)-piperazine-1-carboxylic acid (1H-tetrazol-5-yl)-amide trifluoroacetate
[0159]
[0160] MS: 368.5. 1 H NMR (d 6 -DMSO): 15.51(s, 1H), 10.98(s, 1H), 7.54(s, 2H), 7.33-7.32(m, 1H), 4.29(br s, 4H), 3.58-2.86(m, 6H) .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com