ITGB4BP and derivates thereof used for preventing and/or treating hypertrophic scar and fibrosis lesion
A technology for hypertrophic scars and fibrosis, applied in gene therapy, skin diseases, drug combinations, etc., and can solve problems such as unreported effects and functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1. Construction of Shuttle Vectors
[0054] 1.1 Acquisition of target gene sequence
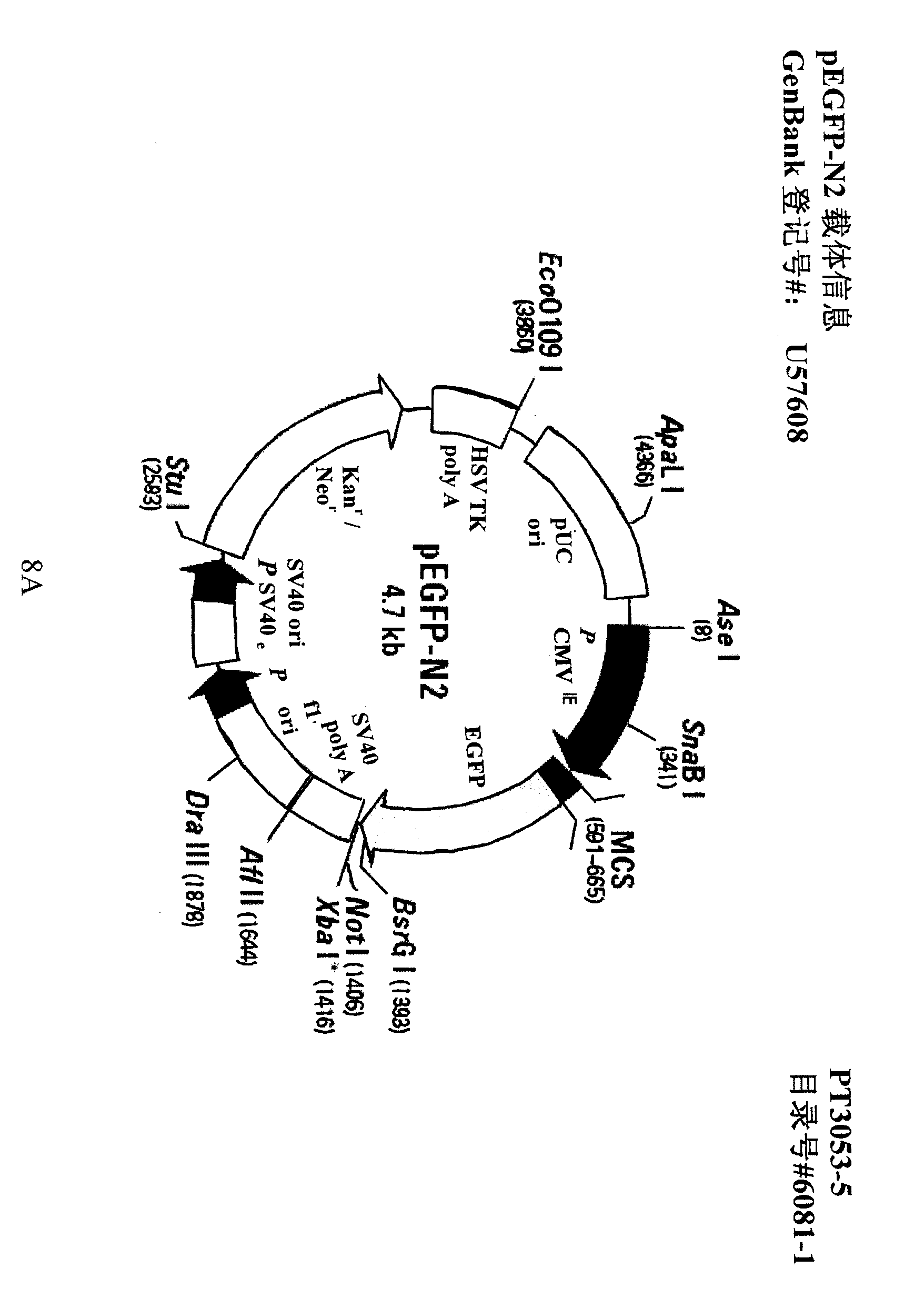
[0055] This experiment was carried out using pEGFP-N2 and pITGB4BP-EGFP plasmids, among which pEGFP-N2 was purchased from clontech company (product number: 6081-1), and pITGB4BP-EGFP was a plasmid constructed in our laboratory. The construction method of pITGB4BP-EGFP is: design primers according to the sequence of the recombinant vector pACT2-ITGB4BP (liver cDNA library, BD Clontech, USA):
[0056] Upstream primer pITGB4BP-EGFP-EcoRI (inserted into the EcoRI restriction site):
[0057] 5'-CAGAATTCATGGCGGTCCGAGCTTCGTT-3';
[0058] Downstream primer pITGB4BP-EGFP-BamHI (inserted into BamHI restriction site):
[0059] 5'-CAGGATCCCGGTGAGGCTGTCAATGAGGGAAT-3'.
[0060] The PCR reaction was carried out with pACT2-ITGB4BP as the template, and the reagents were products of TaKaRa Company. The reaction system is as follows:
[0061] pACT2-ITGB4BP 1μl (about 1μg)
[0062] 10×PCR b...
Embodiment 2
[0109] Example 2. Homologous recombination of adenovirus
[0110] The successfully constructed shuttle vector in Example 1 was recovered after digested with Pme I, and simultaneously transformed into competent Escherichia coli BJ5183 containing the pAdEasy-1 plasmid (purchased from Qbiogene, USA) prepared by the calcium chloride method , complete the homologous recombination of adenovirus. The specific operation content is as follows:
[0111]2.1 Linearization of the shuttle plasmid:
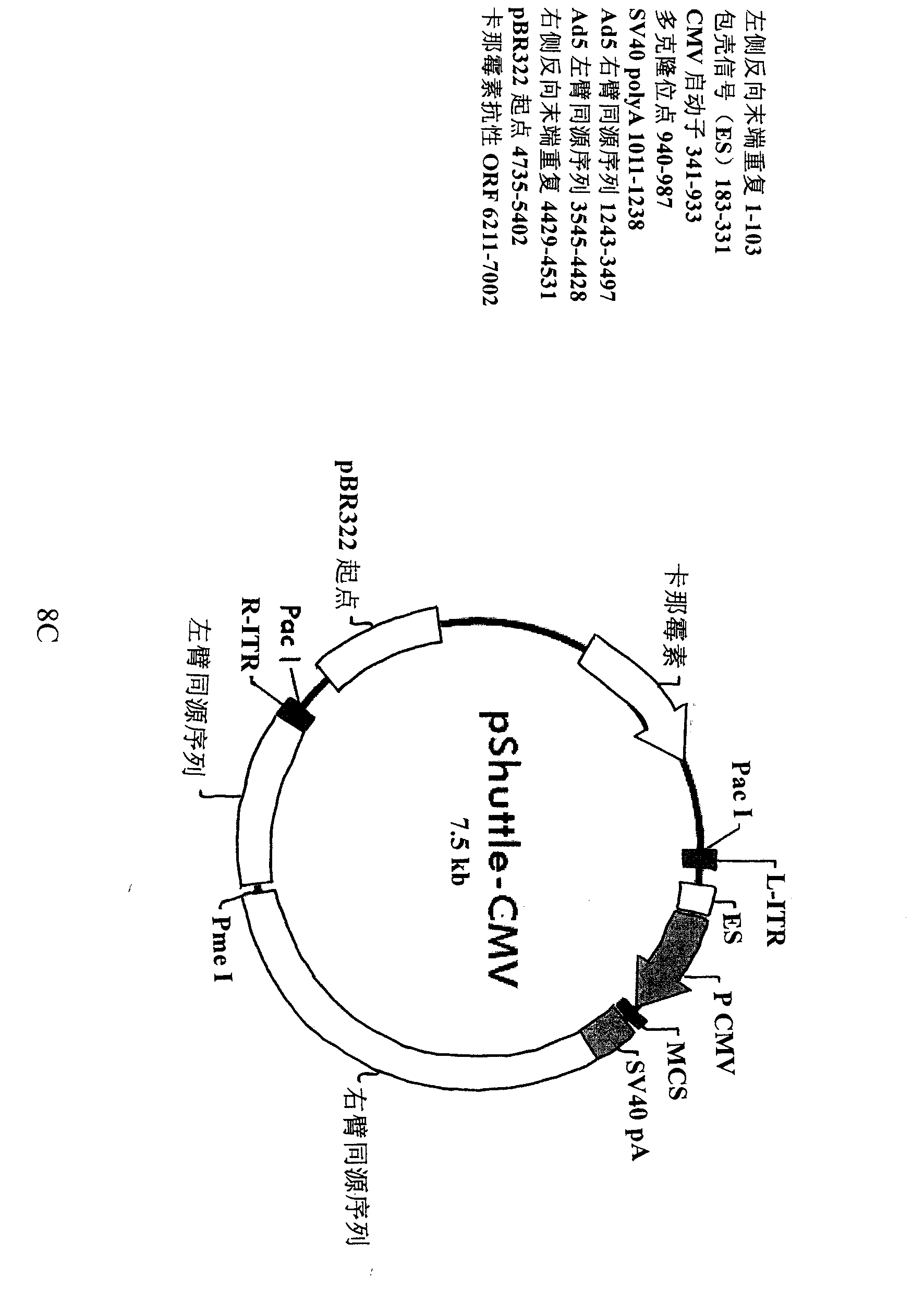
[0112] The successfully constructed shuttle plasmids pShuttle-CMV-ITGB4BP-EGFP and pShuttle-CMV-EGFP were digested and recovered with Pme I (purchased from NEB, UK). The respective enzyme digestion systems of the two are as follows:
[0113] pShuttle-CMV-ITGB4BP-EGFP or pShuttle-CMV-EGFP 18μl (about 0.5μg)
[0114] NEB buffer 4 5 μl
[0115] 100×BSA 0.5 μl
[0116] wxya 2 O 25.5 μl
[0117] Pme I 1 μl
[0118] Final volume 50μl
[0119] Reaction conditions: after 2 hours of reaction at...
Embodiment 3
[0129] Example 3. Packaging and amplification of adenovirus
[0130] 3.1 Adenovirus vector transfection HEK293 cells packaging adenovirus
[0131] 3.1.1 Linearization of adenoviral vectors:
[0132] Recombinant adenoviral vectors pAdEasy-ITGB4BP-EGFP and pAdEasy-EGFP were digested with Pac I and recovered. The enzyme digestion system is as follows:
[0133] pAdEasy-ITGB4BP-EGFP or pAdEasy-EGFP 48μl (about 1.0μg)
[0134] NEB buffer 1 8 μl
[0135] 100×BSA 0.8μl
[0136] wxya 2 O 21.7 μl
[0137] Pac I 1.5μl
[0138] Final volume 80μl
[0139] Reaction conditions: After reacting at 37°C for 1 hour, electrophoresis on 0.6% agarose gel, staining with Goldview nucleic acid dye showed bands of 23kb and 3.0kb or 23kb and 4.5kb respectively. Genes pAdEasy-ITGB4BP-EGFP and pAdEasy-EGFP) were used for gel recovery (the method is the same as 1.1 in Example 1).
[0140] 3.1.2 Packaging of adenovirus:
[0141] will be 3×10 5 Cells HEK293 cells (purchased from the Cell Bank of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com