Prescription for treating allergy purpura and method for preparing same
A technology for allergic purpura and drugs, which is applied in the direction of medical formulas, allergic diseases, and medical preparations containing active ingredients, etc. It can solve problems such as allergic purpura that have not been found, and achieve the effect of relieving clinical symptoms and curing diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
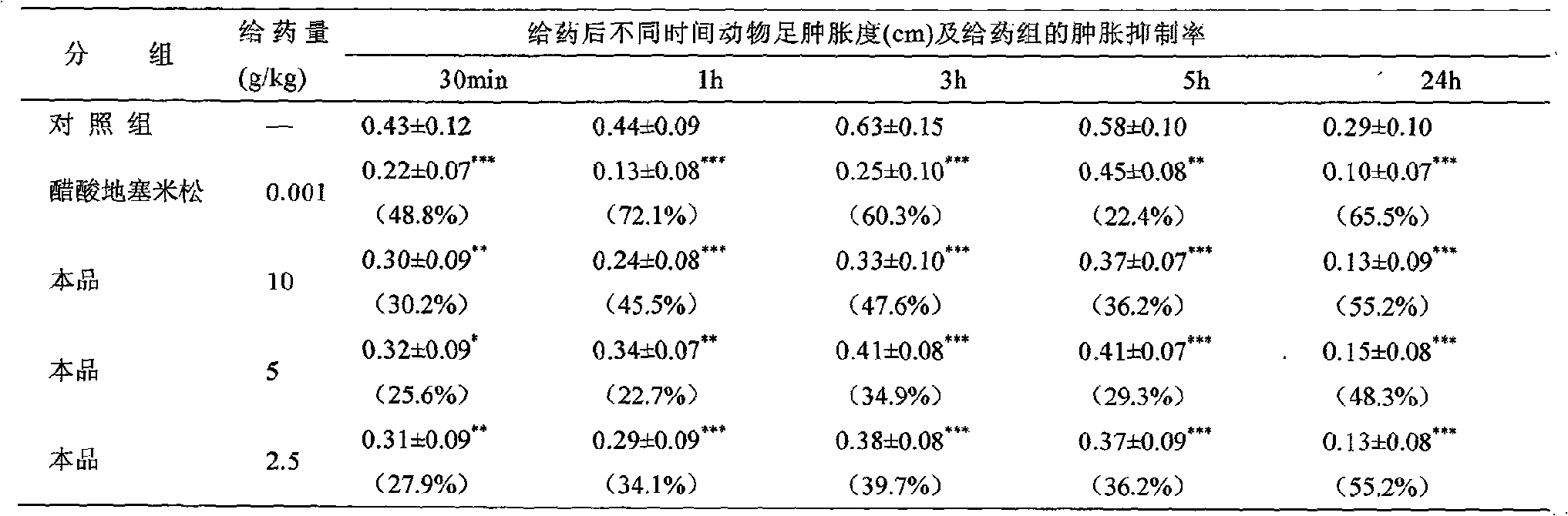
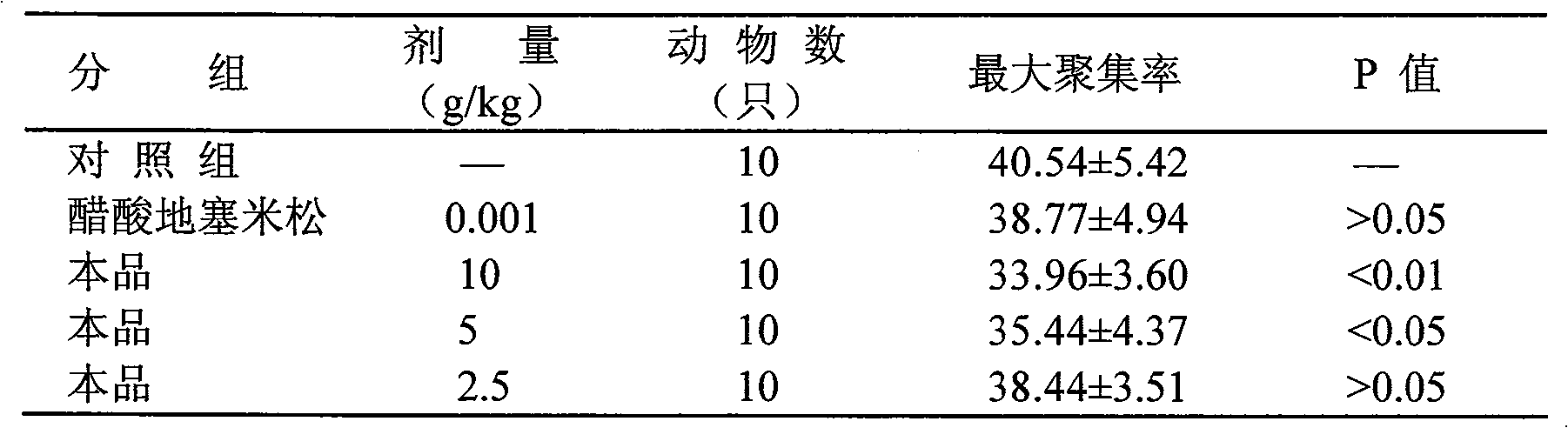
Image
Examples
Embodiment 1
[0073] Embodiment 1 (granule)
[0074] prescription:
[0075] Buffalo horn 500.0g Scutellaria baicalensis 250.0g Comfrey 250.0g
[0076] Moutan bark 250.0g Palm (charcoal) 333.3g Agrimony 250.0g
[0077] Scrophulariaceae 166.7g Honeysuckle 333.3g Forsythia 166.7g
[0078] Sophora flavescens 333.3g
[0079] Preparation method:
[0080] Take comfrey, Moutan cortex, agrimony, and forsythia plus 6 times the amount of 90% ethanol to reflux and extract twice, each time for 1 hour, filter, combine the filtrates, recover ethanol under reduced pressure (below 60°C), and concentrate to relative density 1.10~1.15 (60°C) extract, set aside; decoct dregs, buffalo horn, baicalin, palm, scrophulariaceae, flavescens with 10 times the amount of water, 1.5 hours each time, filter the decoction, Combine the filtrates, concentrate under reduced pressure (below 80°C) to a relative density of 1.10-1.15 (80°C) extract, and save for later use; take honeysuckle and add 10 times the amount of wate...
Embodiment 2
[0081] Embodiment 2 (mixture)
[0082] prescription:
[0083] Buffalo horn 500.0g Scutellaria baicalensis 250.0g Comfrey 250.0g
[0084] Moutan bark 250.0g Palm (charcoal) 333.3g Agrimony 250.0g
[0085] Scrophulariaceae 166.7g Honeysuckle 333.3g Forsythia 166.7g
[0086] Sophora flavescens 333.3g
[0087] Preparation method:
[0088]Take comfrey, Moutan cortex, agrimony, and forsythia plus 6 times the amount of 90% ethanol to reflux and extract twice, each time for 1 hour, filter, combine the filtrates, recover ethanol under reduced pressure (below 60°C), and concentrate to relative density 1.10~1.15 (60°C) extract, set aside; decoct dregs, buffalo horn, baicalin, palm, scrophulariaceae, flavescens with 10 times the amount of water, 1.5 hours each time, filter the decoction, Combine the filtrates, concentrate under reduced pressure (below 80°C) to a relative density of 1.10-1.15 (80°C) extract, and save for later use; take honeysuckle and add 10 times the amount of water...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com