Epoxidation method of gel-free double bond-contained polymer
A polymer and epoxidation technology, which is applied in the field of epoxidation of gel-free polymers containing double bonds, can solve the problem of affecting the economic benefits of the epoxidation process, slowing down the epoxidation reaction speed, and increasing the amount of hydrogen peroxide And other problems, to achieve high yield, high epoxy value, good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
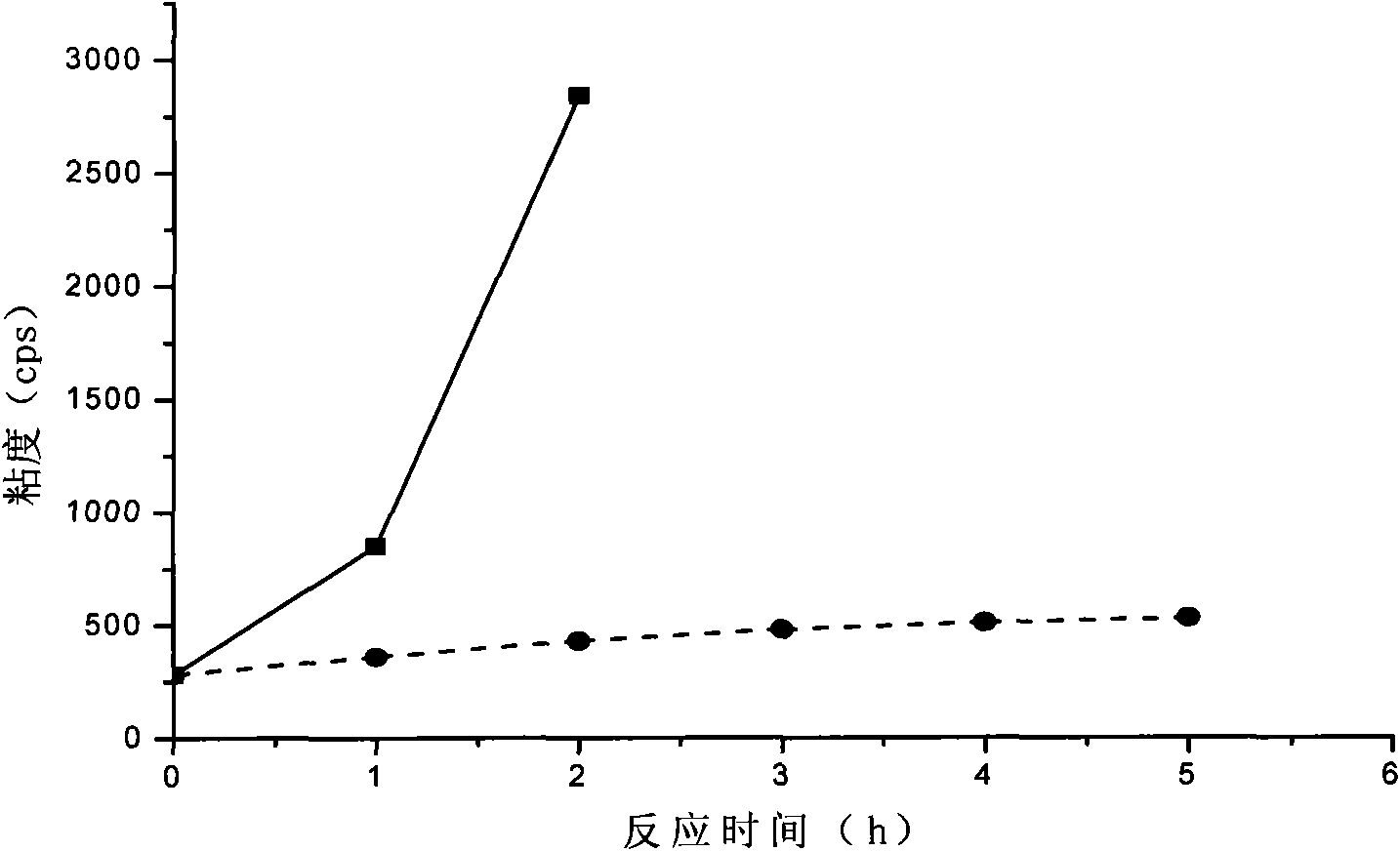
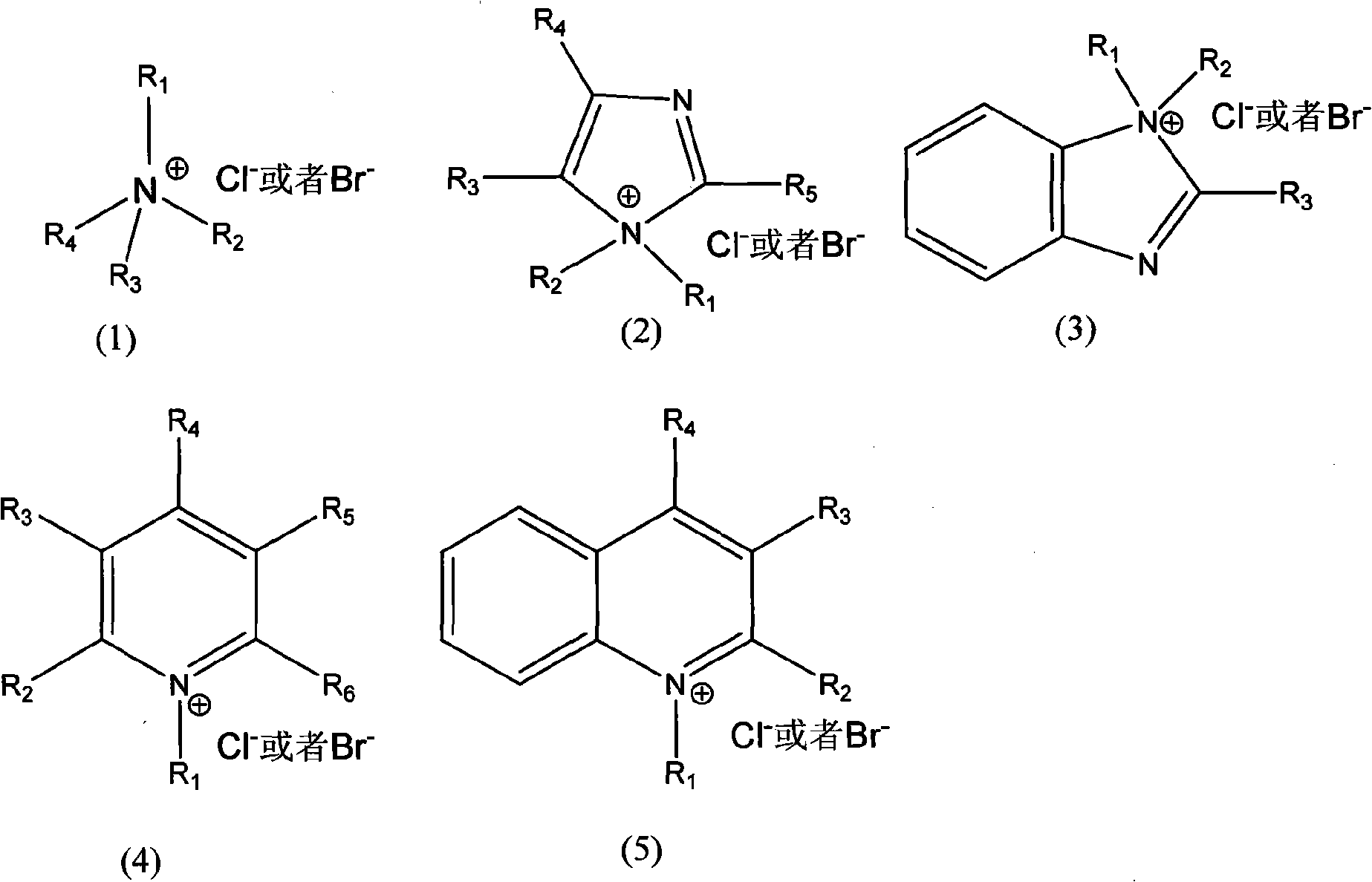
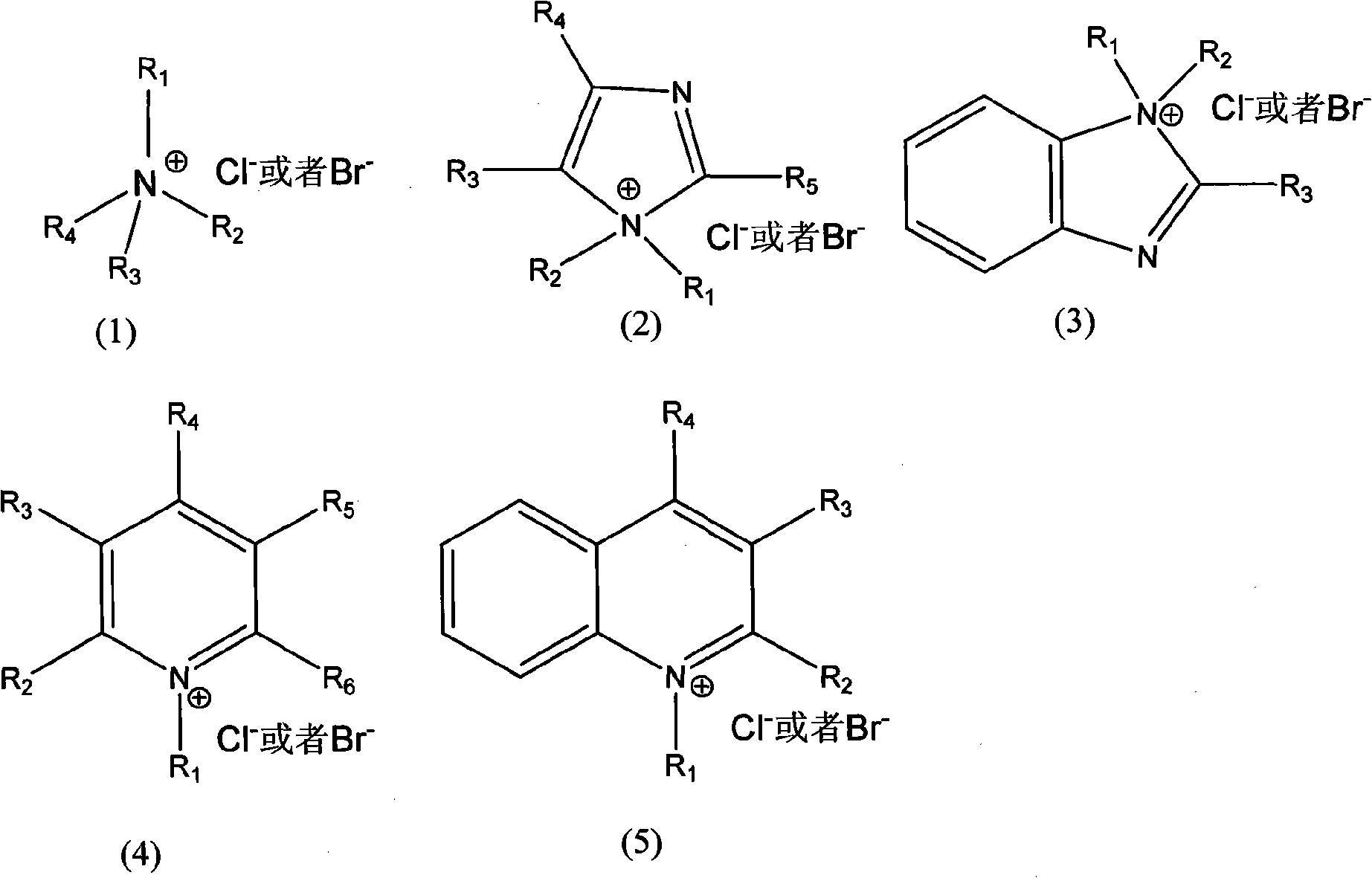
[0021] Dissolve styrene-butadiene rubber (Mn=3.5×10 5 ) to form a 15%wt styrene-butadiene rubber solution. Take 100g of the above-mentioned styrene-butadiene rubber solution, add 0.3g of benzyltrimethylammonium chloride, 7.6mL of formic acid, form a uniform solution, heat up to 40°C, and slowly add 10mL of 30% (v / v) hydrogen peroxide dropwise. Insulate at 40°C for 1 hour, then raise the temperature to 60°C for 5 hours. After the reaction, wash with deionized water four times, separate the organic layer with a separatory funnel, and remove toluene under reduced pressure to obtain epoxidized styrene-butadiene rubber.
[0022] The double bond conversion rate of epoxidized styrene-butadiene rubber was 76.3%, and the extraction residual rate was 1.34%.
Embodiment 2
[0024] Butadiene rubber (Mn=1.1×10 5 ) to form a 10%wt butadiene rubber solution. Take 100g of the above solution, add 1.2g of N-dimethylimidazole chloride and 4.5mL of formic acid to form a homogeneous solution, heat up to 40°C, and slowly add 20mL of 15% (v / v) hydrogen peroxide dropwise. Insulate at 40°C for 2h, then raise the temperature to 65°C for 4h. After the reaction, wash with deionized water for 4 times, separate the organic layer with a separatory funnel, and remove the cyclohexane under reduced pressure to obtain epoxidized butadiene rubber.
[0025] The double bond conversion rate of epoxidized butadiene rubber was 81.3%, and the extraction residual rate was 3.26%.
Embodiment 3
[0027] Dissolve natural rubber with 1,2 dichloroethane (Mn=1.27×10 6 ) to form a 20%wt solution. Then take 100g of the above solution, add 0.4g of N-dimethylbenzimidazole chloride and 3.4mL of formic acid to form a uniform solution, then heat up to 40°C, and slowly add 22mL of 15% (v / v) hydrogen peroxide dropwise. Insulate at 40°C for 1h, then raise the temperature to 65°C for 6h. After the reaction, wash with deionized water for 3 times, separate the organic layer with a separatory funnel, and remove the cyclohexane under reduced pressure to obtain epoxidized natural rubber.
[0028] The double bond conversion rate of epoxidized natural rubber was 85.4%, and the extraction residual rate was 2.83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com