Method for preparing nimesulide intermediate 2-phenoxymethanesulphonylaniline
A technology of phenoxymethane and sulfonanilide, which is applied in the field of preparation of non-steroidal anti-inflammatory drug nimesulide, can solve problems such as polluted air, foul smell of pyridine, and long preparation time, so as to save reaction time, Shorten the reaction steps and avoid the effect of recrystallization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
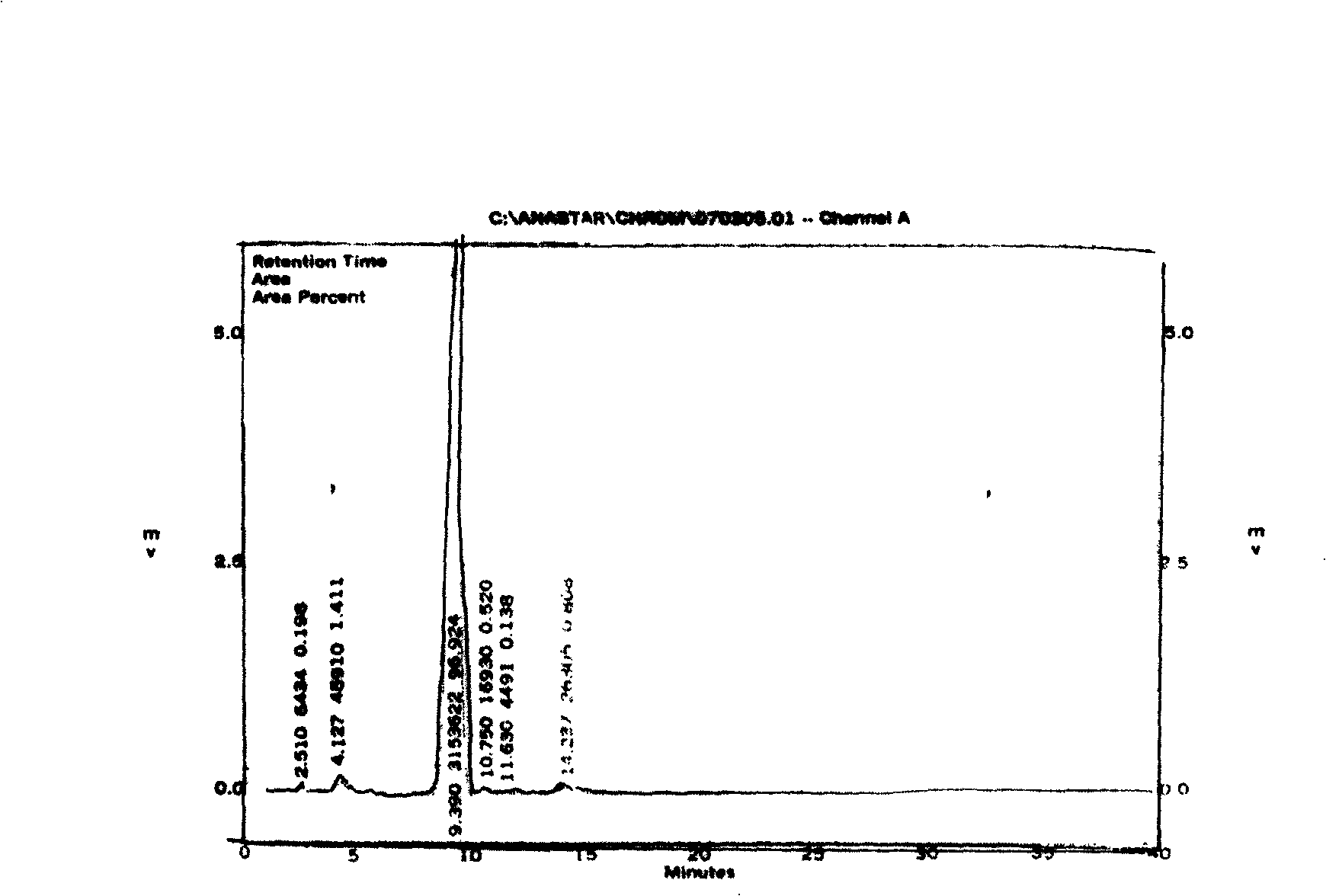
Image
Examples
Embodiment Construction
[0011] The present invention will be further described below in conjunction with embodiment, but does not limit the present invention.
[0012] Example 1. Add 1.6L of ethyl acetate solution containing 1685 grams of o-aminodiphenyl ether to a three-neck flask equipped with mechanical stirring and a drying tube of 5 L. Add 1029 grams of methanesulfonyl chloride dropwise at 88 ° C. The addition is completed in one hour. Continue Respond for an hour. The mixture was poured into ice water, stirred continuously, filtered and dried to obtain 2-phenoxymethanesulfonanilide. The HPLC content was 93.1%, and the yield was 91%.
[0013] Example 2. Add 1.6L of ethyl acetate solution containing 1685 grams of o-aminodiphenyl ether to a three-neck flask equipped with mechanical stirring and a drying tube of 5L, add 1029 grams of methanesulfonyl chloride dropwise at 82°C, add it in one hour, continue Respond for an hour. The mixture was poured into ice water, stirred continuously, filtered a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com