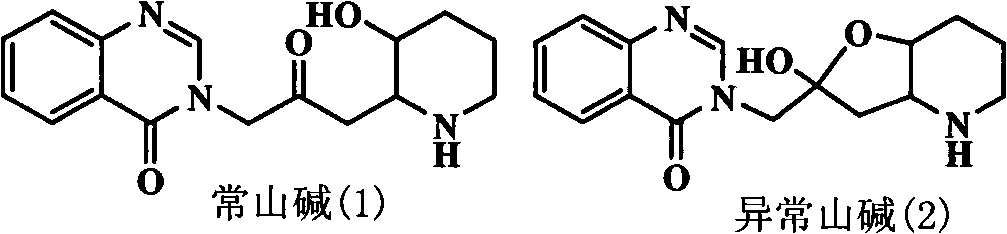
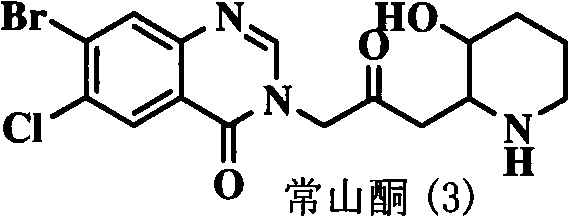
Quinazoline ketone anticoccidial medicament
A kind of quinazolinone, halogenated quinazolinone ring technology, applied in the field of drug synthesis, can solve the problems of high compound price, affecting product use and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: Synthesis of 6-bromo-3-[4-(2-methoxyphenyl)-2-butanonyl]-4-(3H)-quinazolone
[0026] Add 2.00g (0.0089mol) 6-bromo-quinazolin-4-one, 1.0gKI, 0.43g (0.0178mol) NaH, 30ml DMSO in a 50ml three-necked flask, heat up to 75°C, and add dropwise 4.57g ( 0.0178mol) the mixed solution of 1-bromo-4-(2-methoxyphenyl)butan-2-one and 5ml DMSO, stirred and reacted for 1 hour, cooled and added 100ml water, extracted with ethyl acetate, and the organic phase was washed with Dry over sodium sulfate and remove the solvent. The resulting solid is recrystallized from absolute ethanol to obtain a white flocculent solid, which is dried to obtain 1.86 g, with a yield of 52.1%. 1 HNMR (CDCl 3 , 400MHz) d: 8.41(d, J=2.4Hz, 1H), 7.85(m, 1H), 7.74(s, 1H), 7.60(q, J 1 =8.0Hz,J 2 =8.4Hz, 1H), 7.21(dd, J 1 =8.0Hz,J 2 =8.0Hz 1H), 7.15(dd, J 1 =7.2Hz,J 2 =7.6Hz, 1H), 6.90(q, J 1 =7.6Hz,J 2 =8.4Hz, 2H), 4.73(s, 2H), 3.84(s, 3H), 2.98(d, J=6.4Hz, 2H), 2.92(t, J 1 =6.4Hz,J 2 =7.2H...
Embodiment 2
[0027] Example 2: Synthesis of 8-bromo-6-chloro-3-[4-(2-methoxyphenyl)-2-butanonyl]-4-(3H)-quinazolone
[0028] Add 2.31g (0.0089mol) 8-bromo-6-chloro-quinazolin-4-one, 1.0gKI, 0.86g (0.0178mol) NaH, 30ml DMSO into a 50ml three-necked flask, heat up to 75°C, and drop under stirring Add a mixture of 4.57g (0.0178mol) 1-bromo-4-(2-methoxyphenyl)butan-2-one and 5ml DMSO, stir the reaction for 1 hour, add 100ml water under cooling, and extract with ethyl acetate. The organic phase was dried over anhydrous sodium sulfate, and the solvent was removed. The resulting solid was recrystallized from absolute ethanol to obtain a white flocculent solid, which was dried to obtain 2.21 g, with a yield of 57.1%. 1 HNMR (CDCl 3 , 400MHz) δ: 8.15 (d, J 1 =2.0Hz, 1H), 7.95(d, J 1 =2.4Hz, 1H), 7.78(s, 1H), 7.15(dd, J 1 =8.0Hz,J 2 =7.6Hz, 1H), 7.06(d, J 1 =7.2Hz), 6.82(q,J 1 =7.6Hz,J 2 =8.0Hz, 2H), 4.60(s, 2H), 3.78(s, 3H), 2.91(t, J 1 =7.2Hz,J 2 =6.8Hz, 2H), 2.83(q, J1 =6.8Hz,J 2 =7.2H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com