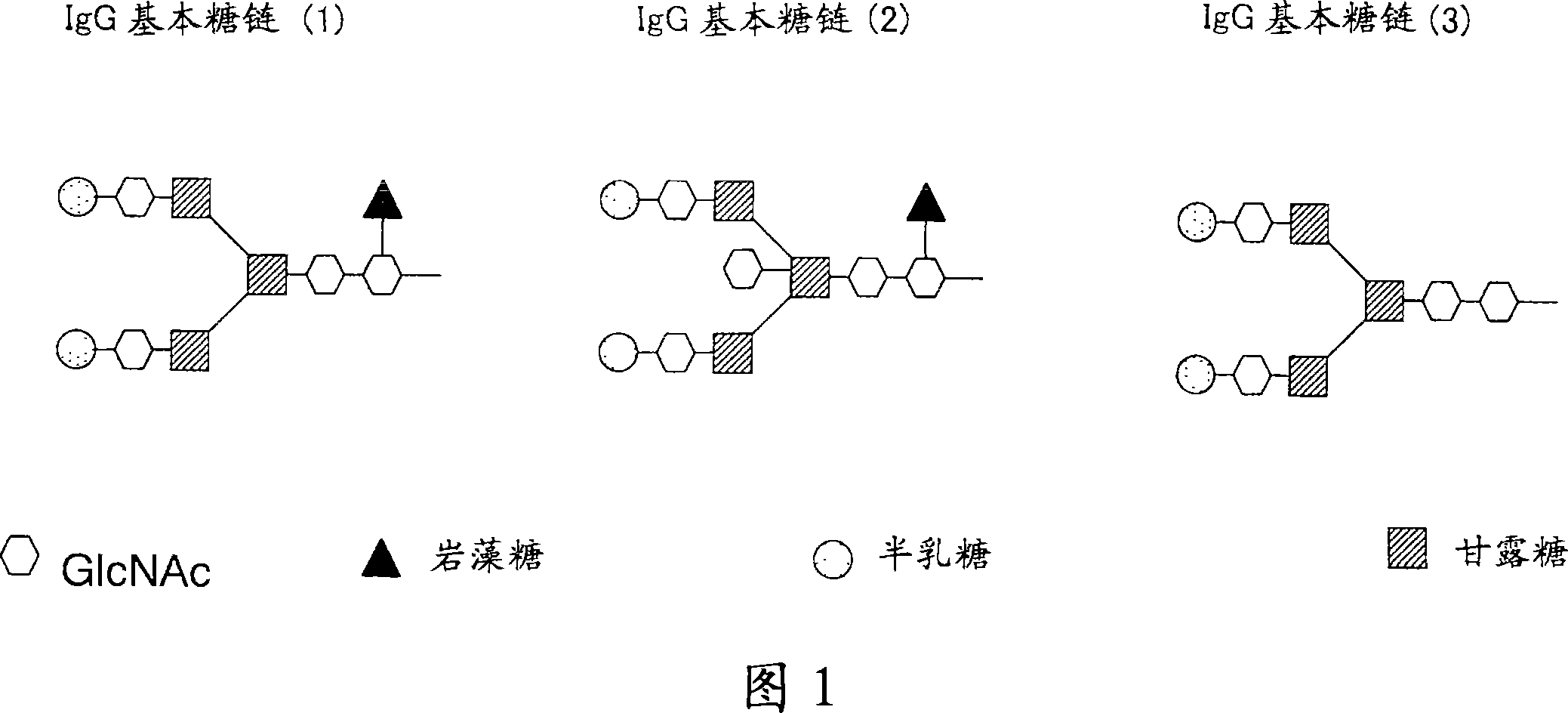
Anti-glypican 3 antibody having modified sugar chain
一种抗磷脂酰肌醇、聚糖的技术,应用在糖链改变的抗磷脂酰肌醇聚糖3抗体领域,能够解决没有公开抗磷脂酰肌醇聚糖3抗体等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Preparation of mouse anti-GPC3 antibody
[0076]A soluble GPC3 protein lacking the C-terminal hydrophobic region (amino acids 564-580) was prepared as an immune protein for preparing anti-GPC3 antibodies, and immunized. Autoimmune disease mice MRL / MpJUmmCrj-lpr / lpr mice (hereinafter referred to as MRL / lpr mice, purchased from Charles River, Japan) were used as immunized animals. Immunization was started when the mice were 7 or 8 weeks old, and the preparation for the primary immunization was adjusted to a dose of 100 μg / mouse of soluble GPC3. Emulsions were prepared using Freund's complete adjuvant (FCA, Becton Dickinson) and injected subcutaneously. After 5 times of immunization, the last immunization dose was diluted with PBS to 50 μg per mouse, and administered intravenously through the tail vein. On the 4th day after the final immunization, spleen cells were taken out, mixed with mouse myeloma cells P3-X63Ag8U1 (hereinafter referred to as P3U1, purchased from ATCC...
Embodiment 2
[0080] Preparation of anti-GPC3 antibody mouse-human chimeric antibody
[0081] The H chain and L chain variable region sequences of anti-GPC3 antibody GC33 were linked to the human IgG1 and κ chain constant region sequences. PCR was performed using a synthetic oligonucleotide complementary to the 5' terminal nucleotide sequence of the antibody H chain variable region having a Kozak sequence and a synthetic oligonucleotide having an NheI site complementary to the 3' terminal nucleotide sequence . The obtained PCR product was cloned into a pB-CH vector in which the human IgG1 constant region was inserted into pBluescript KS+ vector (Toyobo Co., Ltd.). The variable region of the mouse H chain and the constant region of the human H chain (γ1 chain) are connected through an NheI site. The prepared H chain gene fragment was cloned into the expression vector pCXND3. In addition, a synthetic oligonucleotide complementary to the 5' terminal nucleotide sequence of the antibody L cha...
Embodiment 3
[0084] Preparation of Low Fucose Anti-GPC3 Chimeric Antibody
[0085] First, YB2 / 0 (ATCC, CRL-1662) cells were used as host cells and cultured in RPMI1640 medium containing 10% FBS. Then by electroporation, under the condition of 1.4kV, 25μF and 7.5×10 6 At a concentration of 1 cell / 0.75 mL PBS(-), 25 μg of the anti-GPC3 chimeric antibody expression vector prepared in Example 2 was introduced into YB2 / 0 cells (ATCCCRT-1662). After a recovery period of 10 minutes at room temperature, the electroporated cells were suspended in 40 mL of RPMI1640 medium containing 10% FBS. A 10-fold dilution was prepared with the same medium, and the cells were dispensed into 96-well culture plates at 100 μL / well. in CO 2 Incubator (5% CO 2 ) for 24 hours, Geneticin (Invitrogen) was added to a concentration of 0.5 mg / mL, and the cells were cultured for 2 weeks. A cell line expressing a high level of the chimeric antibody was screened by sandwich ELISA using an anti-human IgG antibody, and a c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com