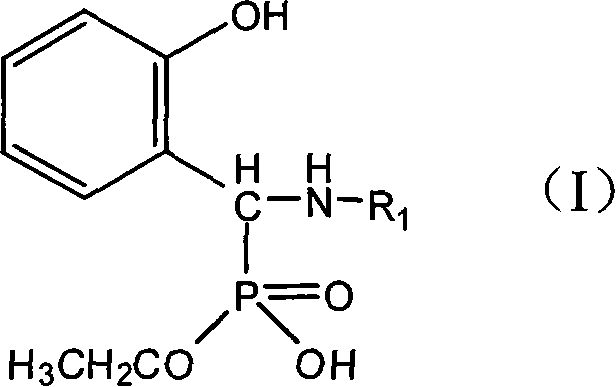
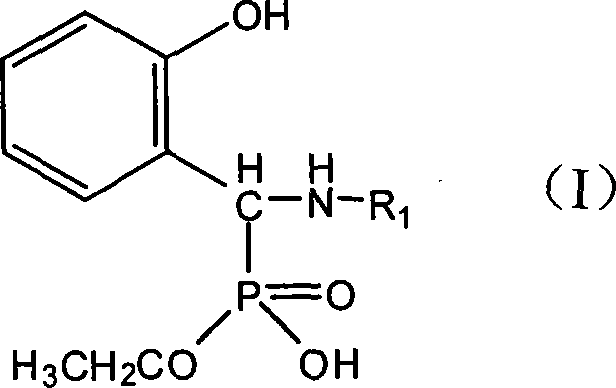
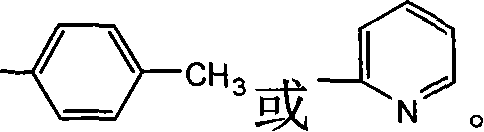Method for synthesizing monoalkyl phosphite
A technology for monoalkyl phosphonite and compounds, which is applied in the field of synthesis of monoalkyl phosphonite compounds, can solve problems such as low yield and harsh conditions, and achieve increased yield, simple method, The effect of improving reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: the synthesis of N-(2-hydroxyethyl) amino-(2'-hydroxyphenyl) methylphosphonic acid monoethyl ester
[0019] Add 0.02 mol of salicylaldehyde and 0.02 mol of ethanolamine into a 100 ml round bottom flask, and reflux for 4 hours under the condition of 30 ml of 95% ethanol as solvent. After adding dropwise a 20ml ethanol solution containing 0.02mol diethyl phosphite, the solution was heated to reflux for 40h. The resulting solid was filtered and washed with ethanol to obtain 2.672 g of a white solid, with a yield of 48.55%.
[0020] The product verification results are as follows:
[0021] 1 H NMR (300MHz, DMSO) δ: 0.97 (CH 3 -,3H), 2.79(-CH 2 -, 2H), 3.49-3.72 (-CH 2 CH 2 -, 4H), 4.64 (-CH-, 1H), 5.33 (-NH-, 1H), 6.84-7.74 (Ar-H, 4H);
[0022] 13 C NMR (300MHz, DMSO) δ: 17.02, 46.77, 54.29, 55.40, 122.72, 95.972, 116.98, 119.73, 129.81, 131.01, 156.50;
[0023] 31 P NMR (300MHz, DMSO) δ: 8.74;
[0024] IR (KBr, cm -1 ): 1604, 1459, 1192, 1077, 104...
Embodiment 2
[0025] Example 2: Synthesis of N-(4-methylphenyl)amino-(2'-hydroxyphenyl)methylphosphonic acid monoethyl ester
[0026] Add 0.02mol of salicylaldehyde and 0.02mol of p-methylaniline into a 100ml round bottom flask, and reflux for 3h under the condition of 30m ethanol as solvent. After dropwise adding 20 ml of ethanol solution containing 0.02 mol of diethyl phosphite, the solution was heated to reflux for 70 h until solid was produced. The resulting solid was filtered and washed with ethanol to obtain 0.932 g of a white solid, with a yield of 14.5%.
[0027] The product verification results are as follows:
[0028] 1 H NMR (300MHz, DMSO): 1.07(-CH 3 , 3H), 2.12(Ar-CH 3 , 3H), 3.88 (O-CH 2 -, 2H), 5.06 (-NH-, 1H), 6.56-7.37 (Ar-H, 8H);
[0029] 13 C NMR (300MHz, DMSO): 16.85, 20.55, 48.16, 62.14, 95.96, 114.55, 119.423, 124.78, 125.60, 128.37, 129.24, 145.60, 155.72;
[0030] 31 P NMR (300MHz, DMSO): 22.12;
[0031] IR (KBr,, cm -1 ): 3394, 1591, 1459, 1221, 1083.
Embodiment 3
[0032] Example 3: Synthesis of N-(2-aminopyridyl)amino-(2'-hydroxyphenyl)methylphosphonic acid monoethyl ester
[0033] Add 0.02 mol of salicylaldehyde and 0.02 mol of 2-aminopyridine into a 100 ml round bottom flask, and reflux for 4 h under the condition of 30 ml of ethanol as solvent. After dropwise adding 20 ml of ethanol solution containing 0.02 mol of diethyl phosphite, the solution was heated to reflux for 36 h until solid was produced. The resulting solid was filtered and washed with ethanol to obtain 2.311 g of a white solid, with a yield of 37.48%.
[0034] The product verification results are as follows:
[0035] 1 H NMR (300MHz, DMSO): 1.06 (CH 3 -,3H), 3.46(-CH 2 -, 2H), 3.74 (-NH-, 1H), 5.20 (-CH-, 1H), 6.56-7.37 (Ar-H, py-H, 8H);
[0036] 13 C NMR (300MHz, DMSO): 16.61, 18.60, 50.33, 56.09, 61.01, 112.05, 117.287, 119.221, 124.575, 128.46, 138.902, 141.894, 154.25, 155.53;
[0037] 31 P NMR (300MHz, DMSO): 16.43.;
[0038] IR (KBr, cm -1 ): 3394, 1591,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com