Novel crystalline polymorphic forms of lercanidipine hydrochloride and process for their preparation
A technology of lercanidipine hydrochloride and lercadipine hydrochloride, applied in solution crystallization, medical preparations containing active ingredients, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
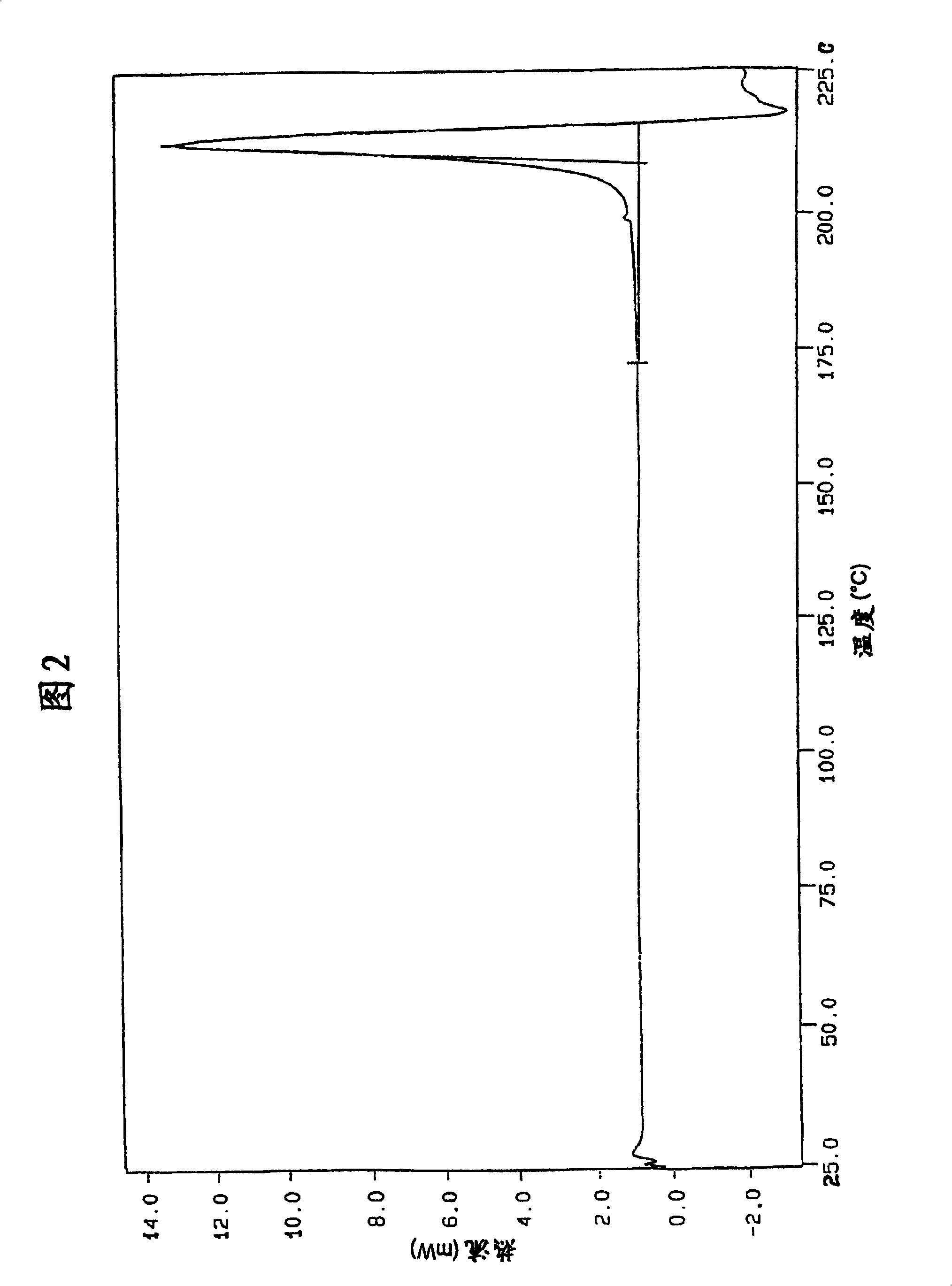
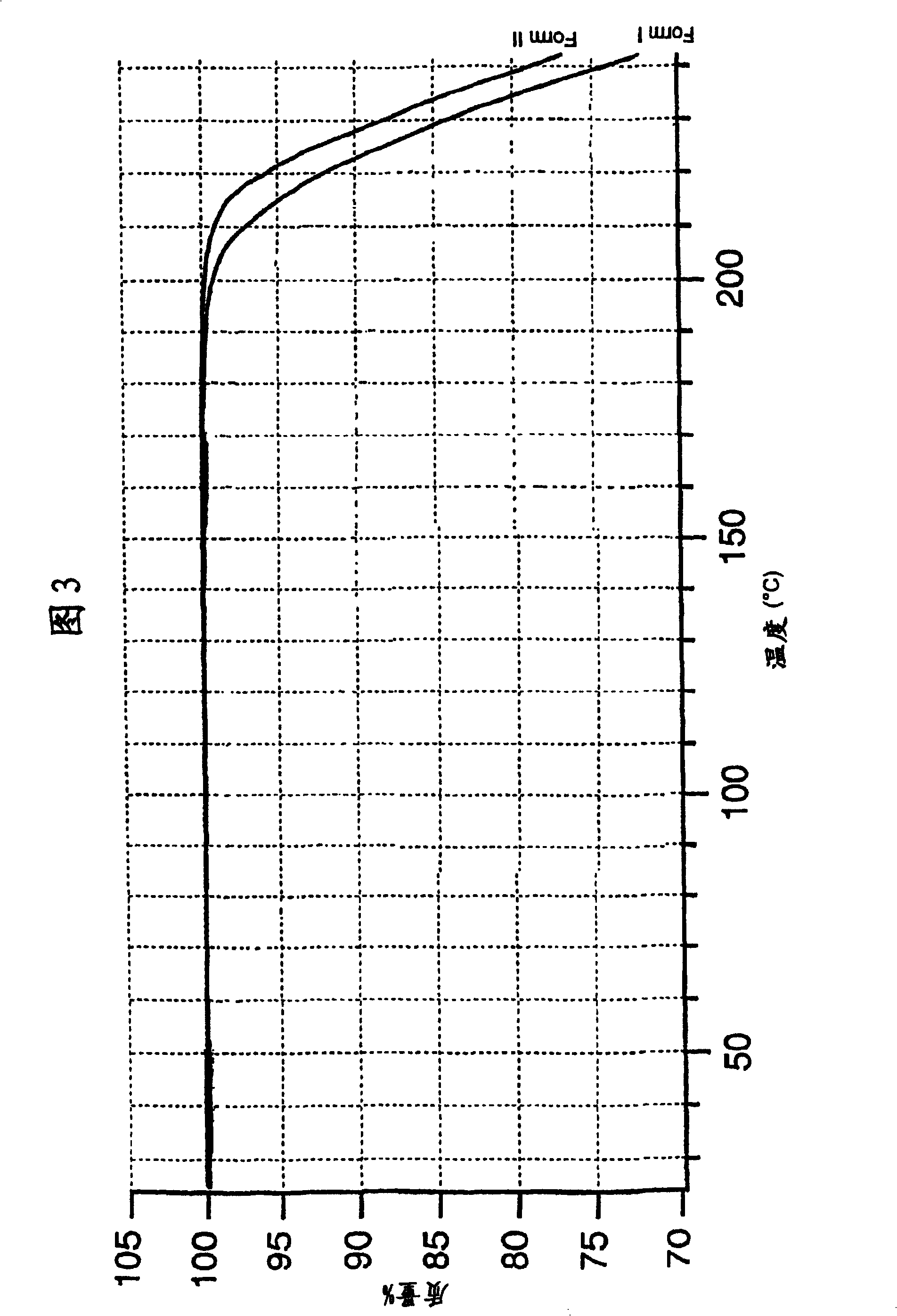
[0157]The following examples for the preparation of lercanidipine hydrochloride crude forms (A) and (B) and crystalline forms (I) and (II), as well as DSC analysis and solubility, stability and hygroscopicity tests are now disclosed for illustrative rather than limiting purposes. Results; Bioavailability tests of the new crystalline form also disclosed.
[0158] Embodiment 1 initial preparation
[0159] Thionyl chloride (36 g) diluted in ethyl acetate (25 g) was slowly added to 2,6-dimethyl-5-methoxycarbonyl-4-( 3-Nitrophenyl)-1,4-dihydropyridine-3-carboxylic acid (90 g in a solution of dimethylformamide (115 g) and ethyl acetate (396 g)). A solution of 2,N-dimethyl-N-(3,3-diphenylpropyl)-1-amino-2-propanol (84 g) in ethyl acetate (72 g) was slowly added to the resulting mixture middle. The whole mixture was kept stirring at the same temperature for 3 hours. The mixture was then heated to 20-25°C and kept under stirring for 12 hours. After this time water (340ml) was adde...
Embodiment 2
[0160] Embodiment 2 thick lercanidipine hydrochloride shape (A)
Embodiment 1
[0161] The organic phase obtained in Example 1 was then azeotropically distilled under a vacuum of about 250 mmHg at a temperature not exceeding 60°C. After removing about 50 ml of water, the solution was concentrated under the same temperature and pressure conditions to about 1 / 3 of the original volume and then neoethyl acetate was added to the original volume until the K.F. value (Karl Fisher value) was about 0.10-0.15% . The final suspension was cooled to 0-5°C. The solid was filtered, suspended in ethyl acetate (350 g), and stirred at 60-65°C for 1 hour. The bulk was cooled to 5-10°C and then filtered. The solid was dried in a 70°C oven. 133 g of dry crude lercanidipine hydrochloride (A) was obtained (75% yield), with a DSC peak of 150-152°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com