Anti-tumor antibody compositions and methods of use
a technology of anti-tumor antibodies and compositions, applied in the field of anti-tumor antibody compositions and methods, can solve the problems of widespread use of psa as a screening tool, disease progression and death, and very limited treatment options for advanced diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experimental examples
VIII. EXPERIMENTAL EXAMPLES
[0280]The above and other features of the invention will now be described more particularly with reference to the accompanying figures and pointed out in the claims. The particular embodiments described below are provided by way of illustration and are not meant to be construed as a limitation on the scope of the invention. It will be apparent to one of ordinary skill in the art that many modifications can be made to the present invention without departing from the spirit or essential characteristics of the invention.
example 1
[0281]The preparation and characterization of the anti-PSCA monoclonal antibodies are described.
I. Materials and Methods
[0282]Hybridomas producing murine and human anti-PSCA monoclonal antibodies (Mabs) were generated. The fusion partner was the mouse myeloma line P3X63Ag8.653 (Kearney, J. F. et al., J. Immunology 123:1548-1550, 1979).
[0283]Table 2A summarizes the immunization strategies and shows the nature of the antigen used for immunization, the adjuvant, dosage, route of administration, dosing schedule and host animal used. Table 2B summarizes the characteristics of the anti-PSCA Mabs as determined by various assays. The antibody names herein are derived from the first 3-4 characters of the hybridoma clone producing the antibody (see Table 2B). The first 3-4 characters before the period in the clone name indicate the parental clone followed after the first period by the name of further subclones, if any. Alternatively, the anti-PSCA antibodies herein are also referred to by the...
example 2
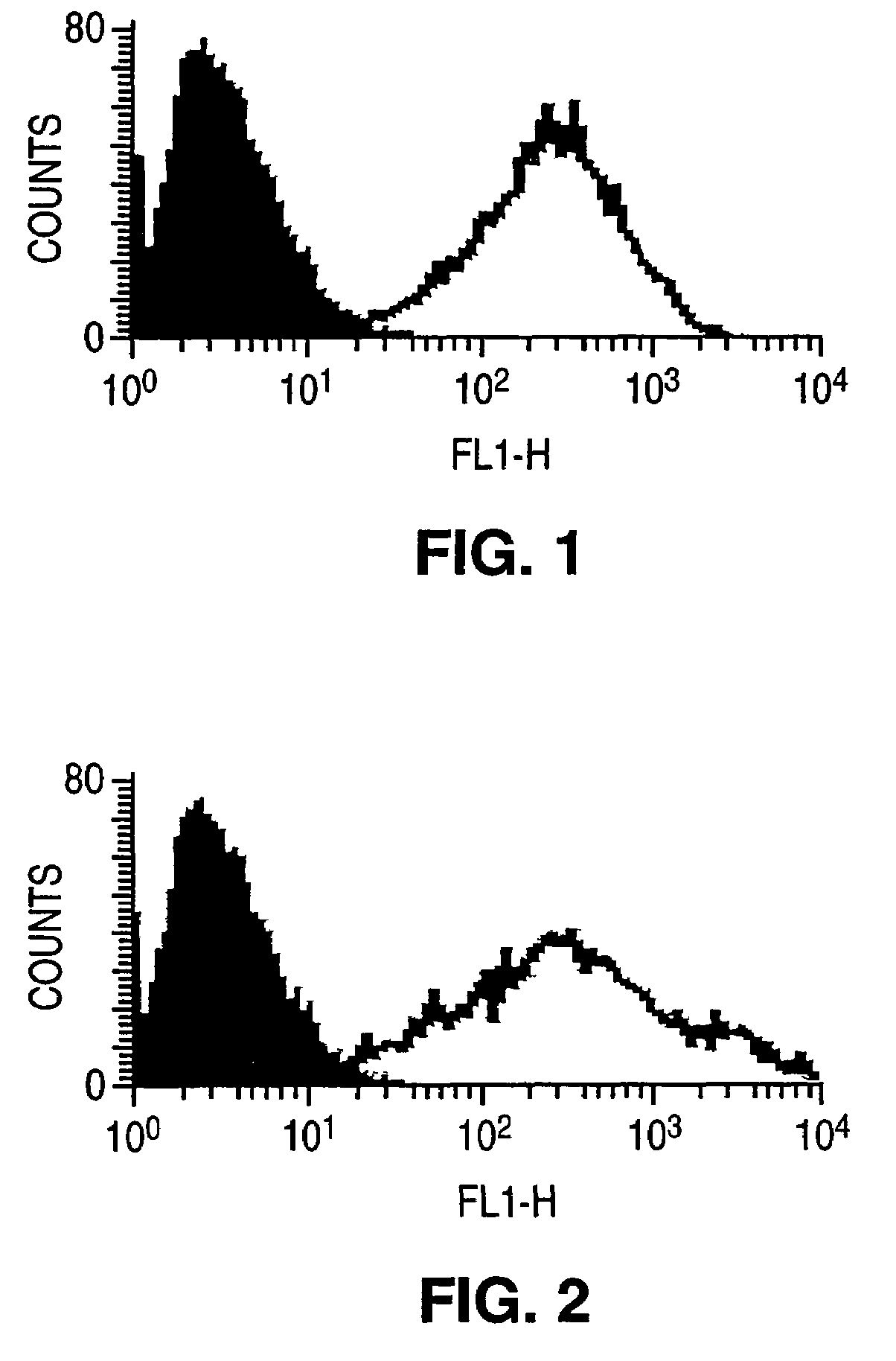
[0307]This example demonstrated the binding and localization of anti-PSCA antibodies 2395 (6F8), 2399 (8D11), 2403 (5F2), 2761 (6B8) [ascites number followed by antibody no. in parenthesis; see Table 2B] to PSCA expressing tumor cells (MCF-7Her2gdPSCA and HCT-gdPSCA) in vivo. By labeling antibodies with the fluorescent dye, Cy3, antibody binding can be visualized by fluorescence microscopy. This technology was well established using Cy3 labeled Herceptin (anti-Her2 monoclonal antibody) in mice bearing Her2-expressing tumors. In vivo binding of the anti-PSCA antibodies were tested on tumor cells transfected with human gd-linked PSCA. Control antibodies included Cy3 labeled Herceptin as a positive control for the MCF-7Her2gdPSCA cells. Additionally, a Cy3 labeled anti-gd antibody, with very good binding activity, was used as a positive control for the PSCA antibodies. Transfected PSCA is gd linked, thus the level of gd protein directly correlates with PSCA protein expression. Cell lin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cytotoxicity | aaaaa | aaaaa |
| Radioactivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com