Methods, Systems, And Compositions For Determining Blood Clot Formation, And Uses Thereof
a technology of blood clot and composition, applied in the field of microfluidic systems, can solve the problems of unreliable and inaccurate current diagnostic and monitoring devices, and the inability to fully incorporate the endothelium in the assessment of the endothelium in clinical laboratories, and achieve the effect of facilitating hemostasis and ensuring reliability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiments a1-a9
A. Embodiments A1-A9
Embodiment A1
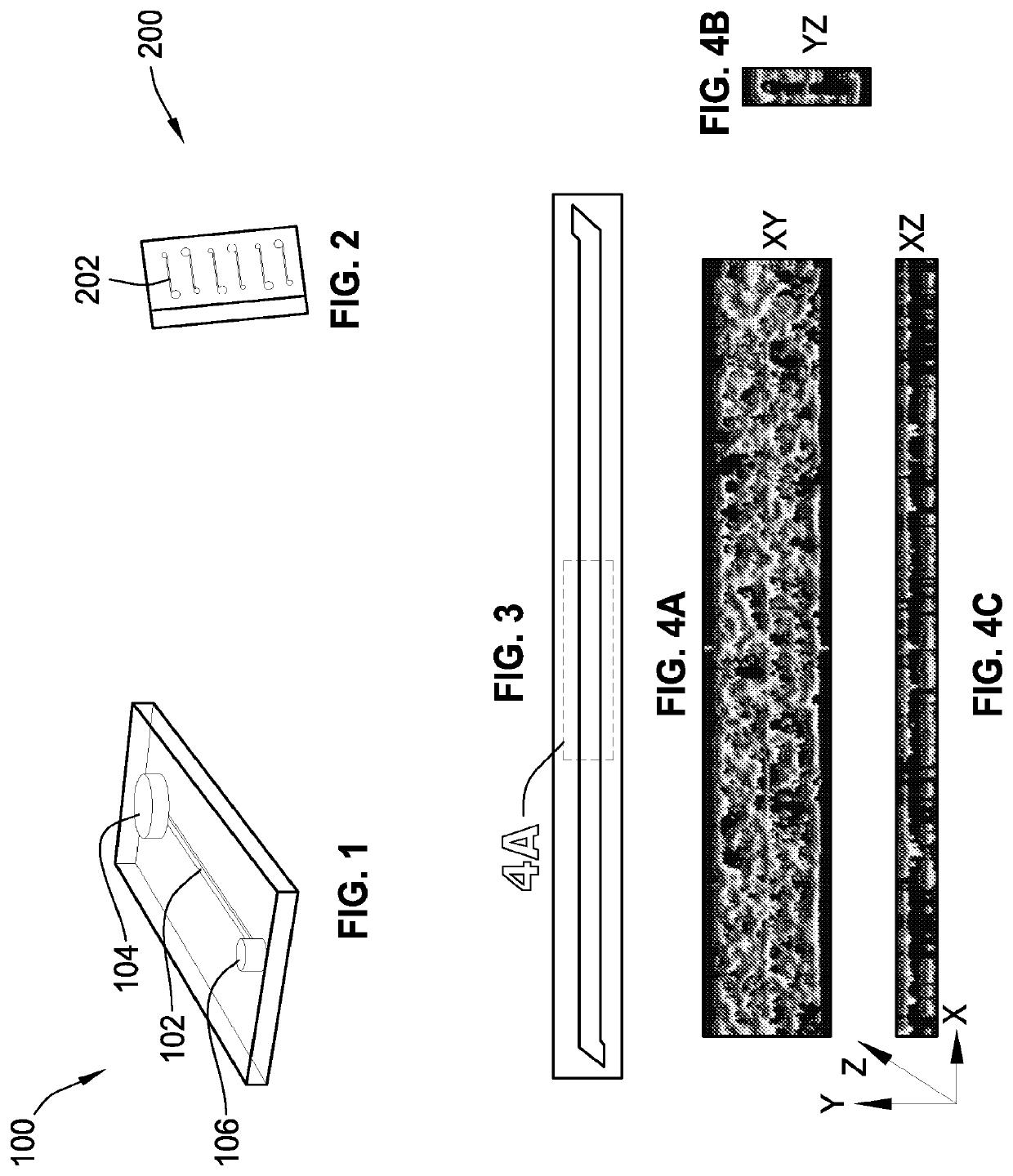
[0422]A microchannel comprising one or more surfaces, the microchannel having living endothelial cells on all of the microchannel surfaces.
Embodiment A2
[0423]The microchannel of embodiment A1, wherein the living endothelial cells are human umbilical vein endothelial cells.
Embodiment A3
[0424]The microchannel of embodiment A1, wherein the surfaces are coated with at least one attachment molecule that supports adhesion of the living endothelial cells.
Embodiment A4
[0425]The microchannel of embodiment A1, wherein the microchannel includes a top surface, a bottom surface, a first side surface, and a second side surface.
Embodiment A5
[0426]The microchannel of embodiment A4, wherein the bottom surface includes a membrane.
Embodiment A6
[0427]The microchannel of embodiment A1, wherein the microchannel is in fluid communication with an input port and an output port.
Embodiment A7
[0428]The microchannel of embodiment A1, wherein the microchannel has a width in the r...
embodiments d1-d8
D. Embodiments D1-D8
Embodiment D1
[0439]A method comprising:
[0440]1) providing[0441]a) a microchannel with one or more surfaces, and[0442]b) living endothelial cells on all of the surfaces; and
[0443]2) introducing fluid into the microchannel.
Embodiment D2
[0444]The method of embodiment D1, wherein the living endothelial cells are human umbilical vein endothelial cells.
Embodiment D3
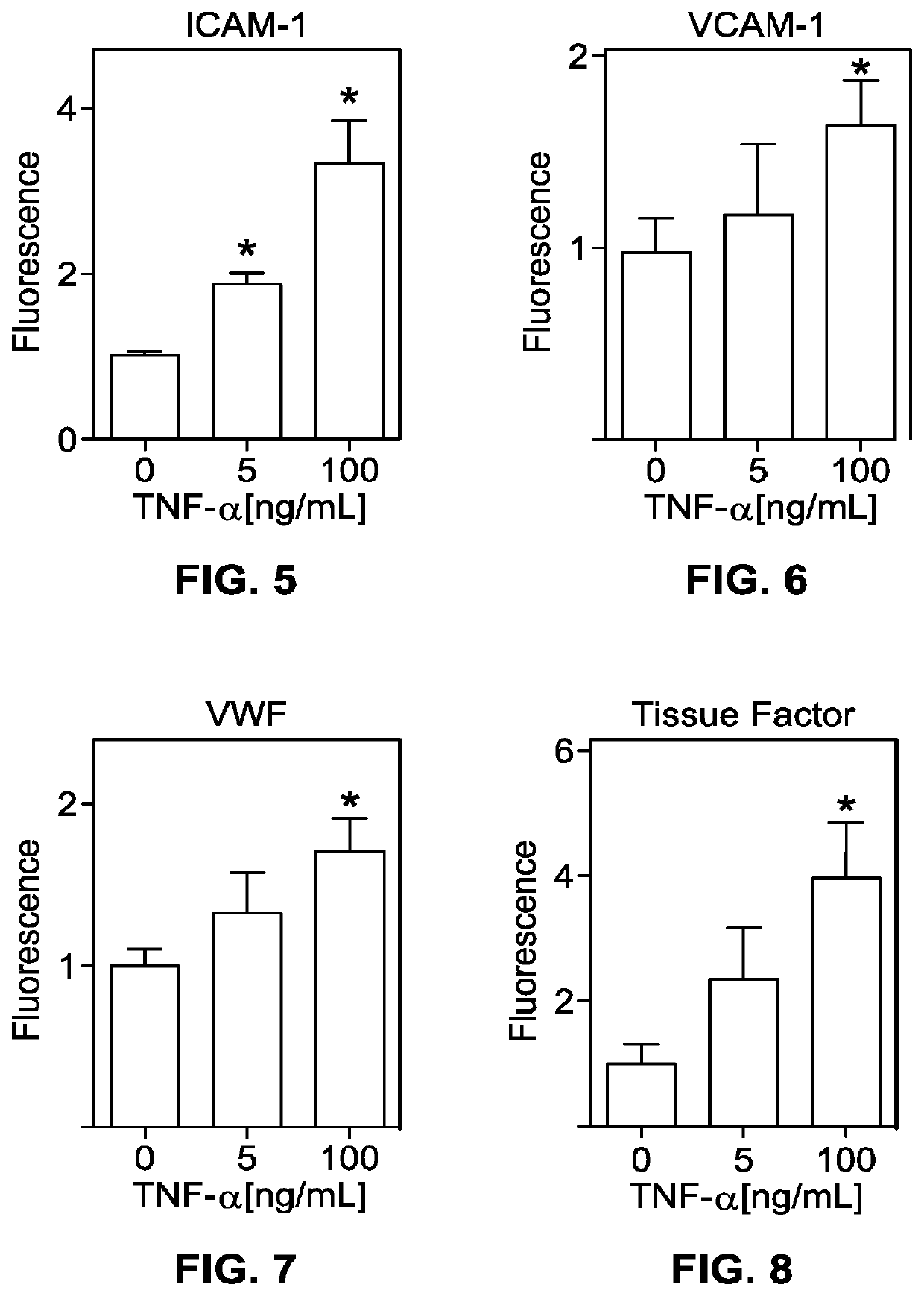
[0445]The method of embodiment D1, wherein the fluid is selected from a group consisting of a blood sample, a serum sample, a plasma sample, a lipid solution, a nutrient medium, or a combination of two or more thereof.
Embodiment D4
[0446]The method of embodiment D1, wherein the fluid includes whole blood that contacts the endothelial cells without clotting.
Embodiment D5
[0447]The method of embodiment D1, wherein the fluid includes platelets, the platelets contacting the endothelial cells without clotting.
Embodiment D6
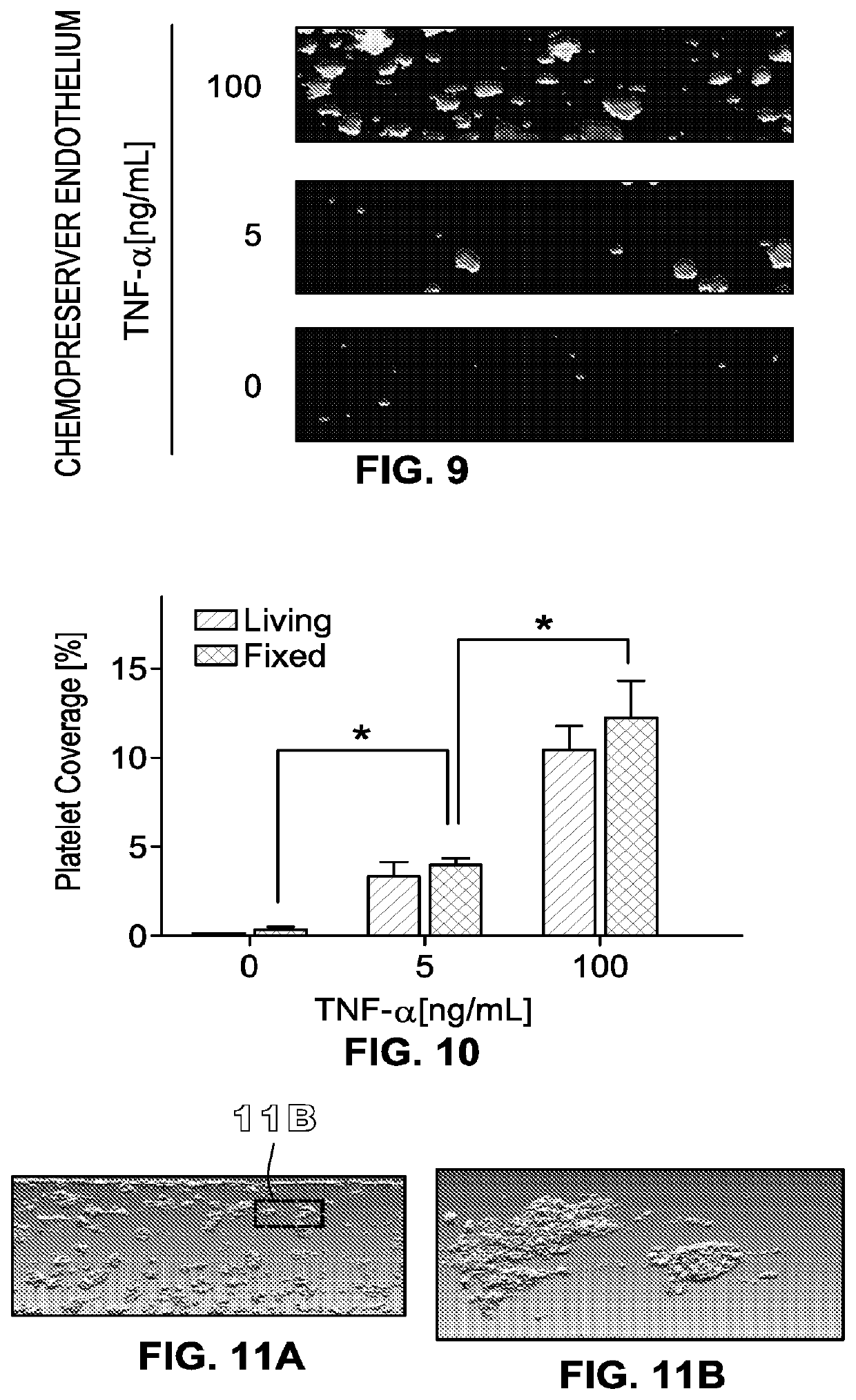
[0448]The method of embodiment D1, further comprising, prior to step 2), exposing the living e...
embodiment d7
[0449]The method of embodiment D6, wherein the fluid includes whole blood that contacts the endothelial ceils under conditions such that a platelet-rich thrombus forms.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com