Conditioning protocols and use of same for tissue regeneration
a tissue regeneration and protocol technology, applied in the direction of artificial cell constructs, drug compositions, unknown materials, etc., can solve the problems of inability to meet the needs of patients, inability to achieve satisfactory/optimal treatments, and only slightly improve the quality of life of patients in need. , the treatment modality suffers from considerable disadvantages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Canalicular ‘Window’ for Human and Mouse Lung Progenitors
Growth Potential of Human Embryonic Lung Tissues Harvested at Different Gestational Time Points
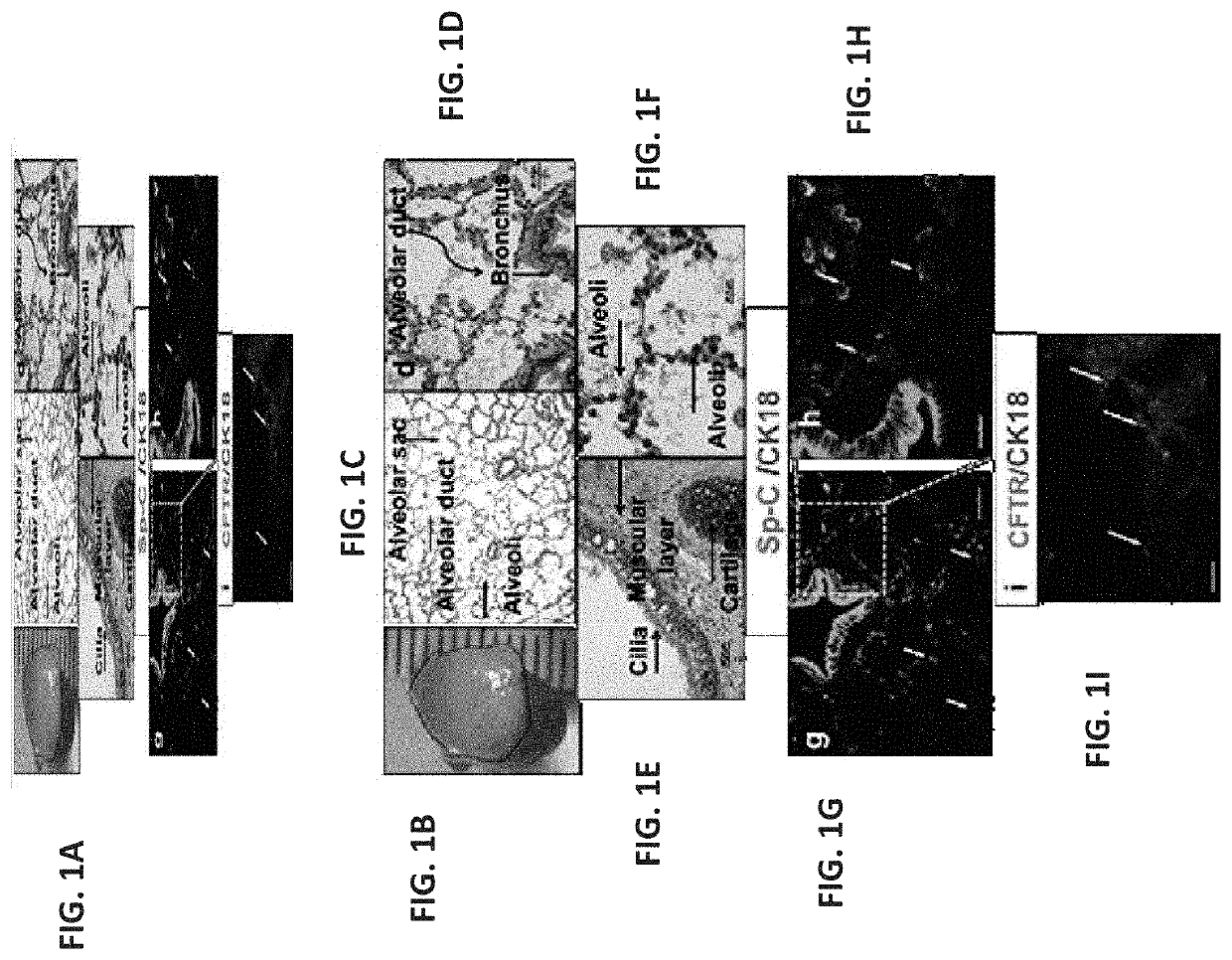
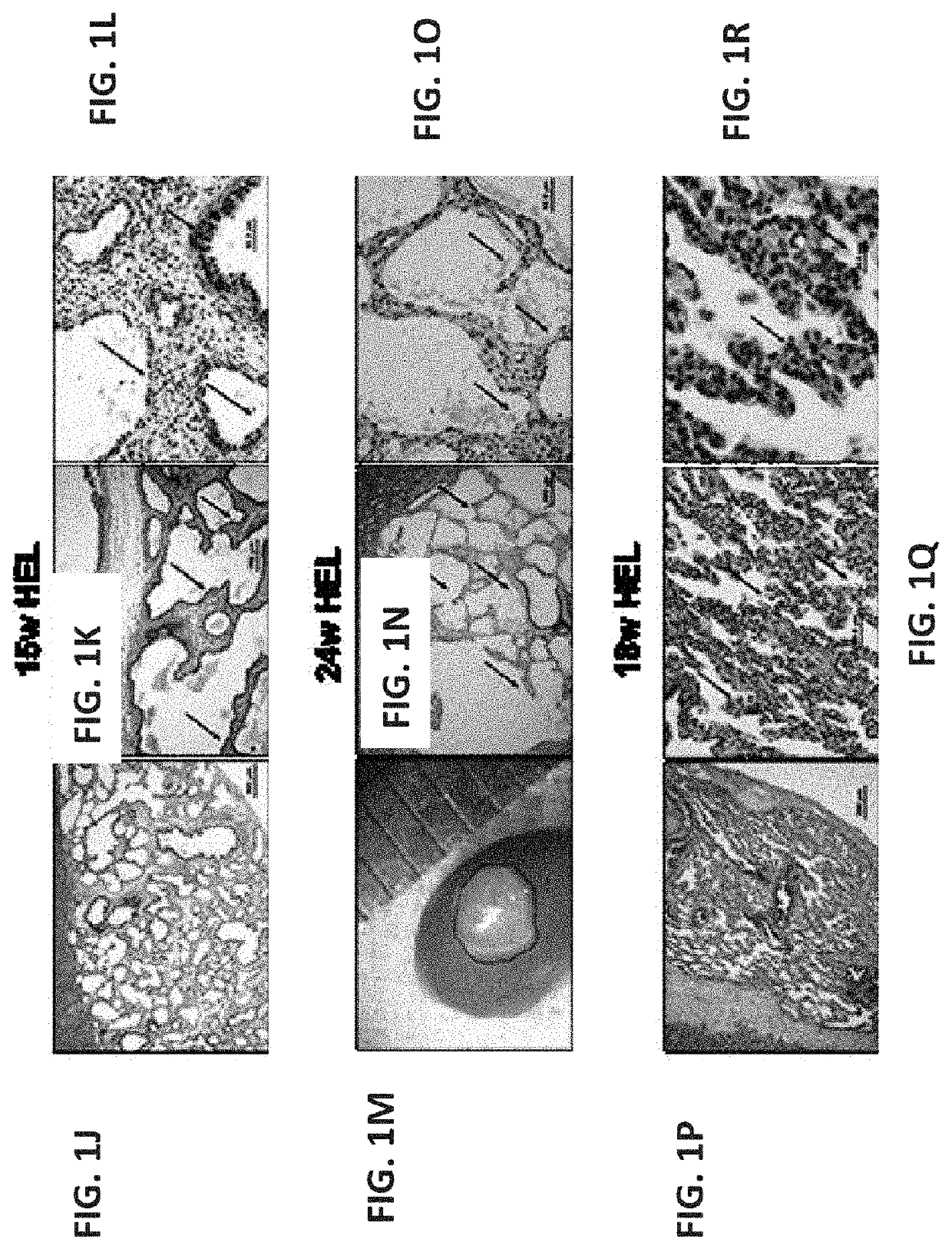
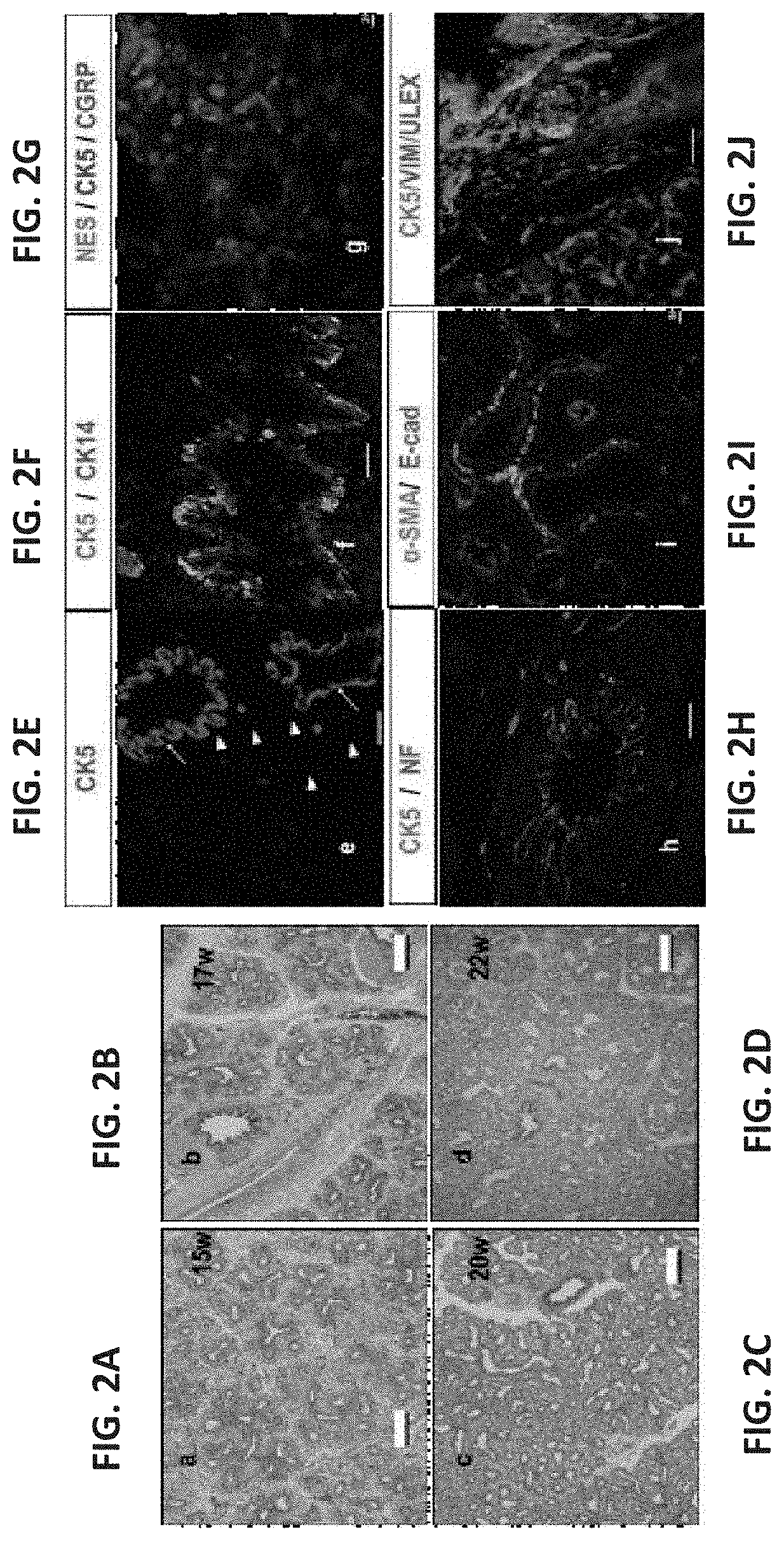
[0391]To evaluate a suitable window for human fetal lung progenitors, the present inventors initially characterized the growth and differentiation potential of lung precursor tissues harvested at different gestational time points (tissues originating from 15- to 24-week human fetuses). Using implantation of whole human (FIGS. 1A-I, FIGS. 26A-B and FIGS. 27A-L) or mouse (data not shown) fetal lung fragments under the renal capsule of immune-deficient (NOD-SCID) or syngeneic mice, respectively, it was found that use of human or mouse lung fetal tissue harvested at the canalicular stage of development enabled optimal growth and differentiation.
[0392]Overall, upon examination at 8 weeks post transplant, more than 98% of the grafts from donor tissue of all ages survived and all recovered grafts demonstrated increased size, with no evidenc...
example 2
Proof of Concept in Mouse Models for the Regenerative Potential of ‘Window’ Embryonic Lung Transplants
Optimal ‘Window’ for Harvesting Mouse Embryonic Lung Precursor Tissue for Transplantation
[0405]In order to assess the curative potential of embryonic lung derived tissue in appropriate mouse models, the optimal “window” for harvesting mouse embryonic lung for transplantation was initially defined, as for its human counterpart. Thus, mouse lung embryonic tissue was harvested at different gestational time points (E14-E17), implanted under the kidney capsule of syngeneic mice, and 8 weeks after transplantation, the implants were assessed for the presence of lung parenchyma, bronchial and alveolar structures, as well as for unwanted presence of fibrosis and cysts.
[0406]As can be seen in FIGS. 10A-E, twelve weeks after sub-capsular renal transplantation, E14 and E17 lung tissue resulted in formation of cystic and fibrotic tissue (FIGS. 10A-B), while E15-E16 mouse embryonic lung exhibited...
example 3
Ablation of Endogenous Lung Progenitors Occupying Niches
[0438]Based on the preliminary results described above, the present inventors tested the curative potential of non-fractionated single cell suspensions of canalicular-staged mouse lung cells, in the well-established NA-induced lung injury model. This model mimics lung diseases caused by epithelial injury, reflected by changes in the presence of pulmonary club cells (formerly referred to as Clara cells, which are marked by staining for Clara cell secretory protein (CCSP; formally known as Scgb1a1). Considering the observation of a marked analogy between the niches of progenitor cells in lung and in the BM, it was tempting to test the i.v. route for cell transfer, commonly used in HSCT. Thus, 2 days following NA administration, recipient C57BL / 6 mice were intravenously infused with 1×106 syngeneic E16 lung cells, derived from GFP-positive embryos. Subsequently, the lungs of the treated animals were assessed at different time poin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com