Peptide YY and Peptide YY Agonists for Treatment of Metabolic Disorders
a metabolic disorder and agonist technology, applied in the field of peptide yy and peptide yy agonists for the treatment of metabolic disorders, can solve the problems of reducing life span, carries a serious risk of co-morbidities, and obesity and its associated disorders are common and serious public health problems, so as to reduce nutrient availability and modulate nutrient availability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
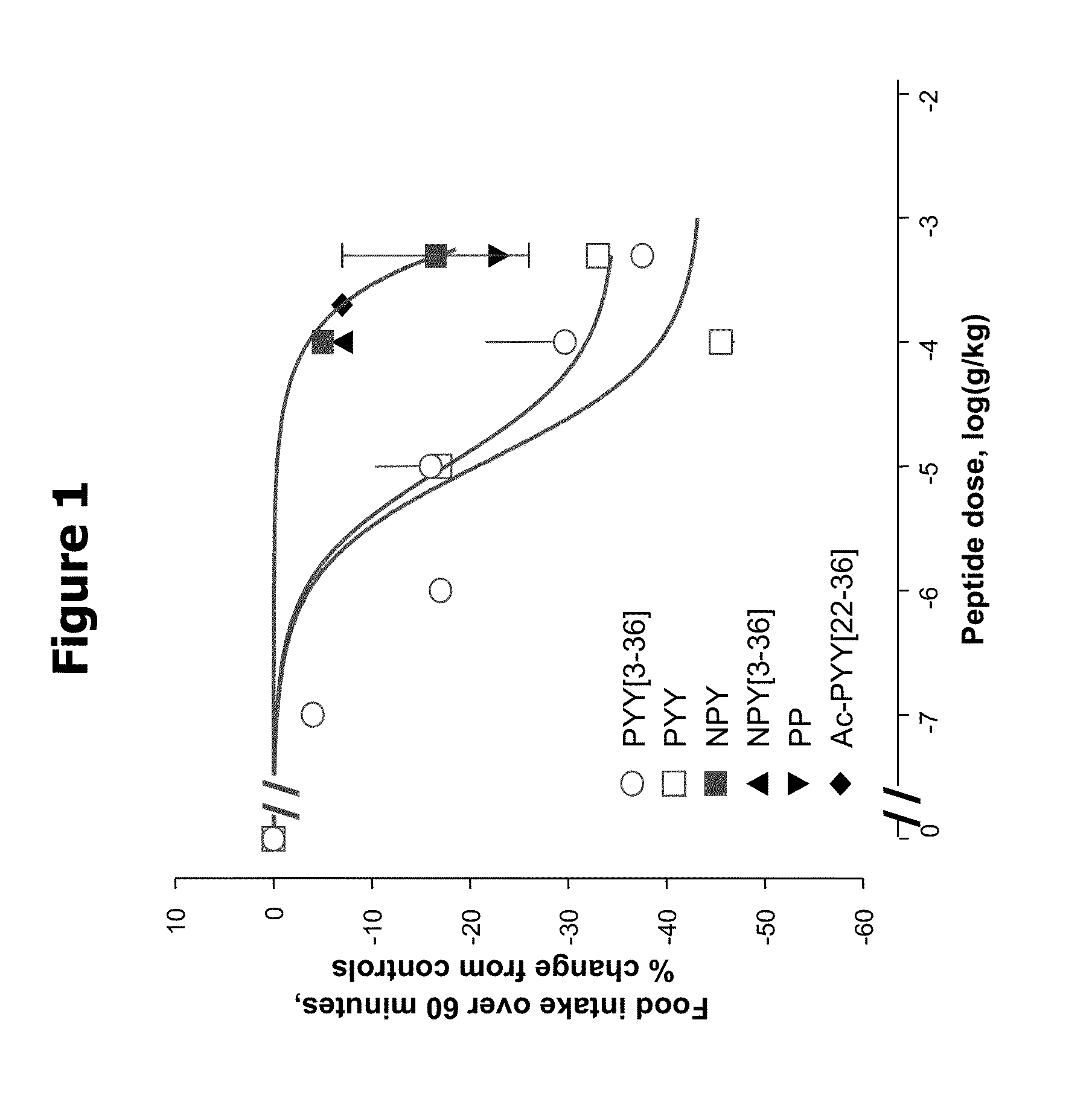
Activity of Y Receptor Ligands on Food Intake in Overnight-Fasted NIH / SW Mice
[0055]Female NIH / Swiss mice (8-12 weeks old) were group housed with a 12:12 hour light:dark cycle with lights on at 0600. Water and a standard pelleted mouse chow diet were available ad libitum, except as noted. Animals were fasted and housed individually starting at approximately 1500 hrs, 1 day prior to experiment. The morning of the experiment (approx. 0630 hrs), all animals were weighed and divided into experimental groups so as to give the most similar weight distribution between groups. In a typical study, n=10 for the control group and at least 5 for each treatment group.
[0056]At time=0 min, all animals were given an intraperitoneal injection of vehicle or compound in a volume of 5 ml / kg and immediately given a pre-weighed amount (10-15 g) of the standard chow. Increasing dosages of PYY[3-36], or PYY (0.1 μg / kg to 500 μg / kg and NPY (100 and 500 μg / kg), and single high doses of NPY[3-36] (100 μg / kg), ...
example 2
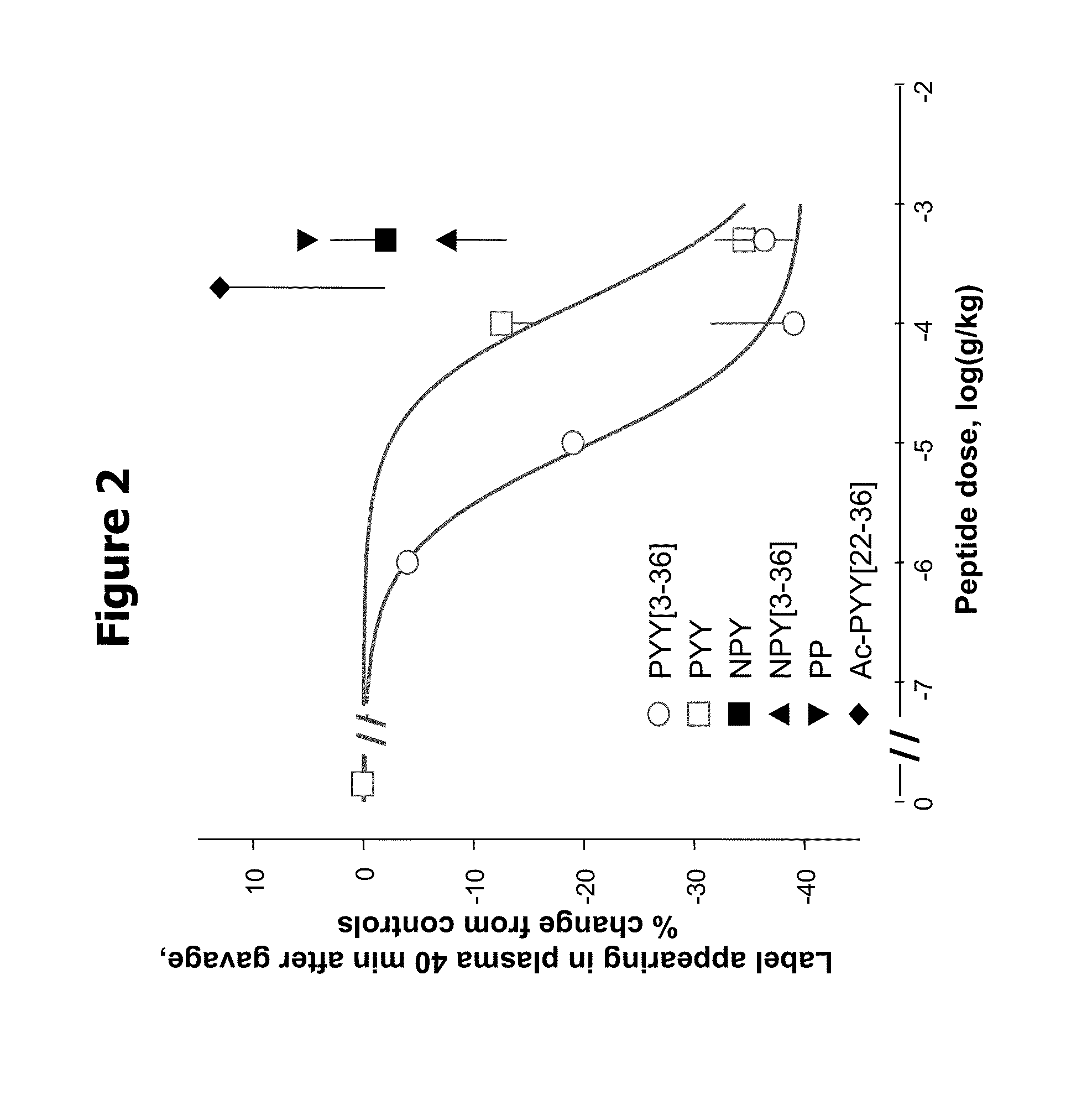
Activity of Y Peptide Ligands on Gastric Emptying in HSD Rats
[0061]Male HSD rats, 180-215 g, were housed with a 12:12 hour light:dark cycle and fasted for 20 hrs (overnight). At time=0 min, test peptide (PYY[3-36], PYY, Ac-PYY[22-36], NPY, NPY[3-36], or PP) or saline vehicle was injected (intraperitoneal) into conscious rats (n=6 / group). At t=1 min, a solution of 1 mL sterile water containing 5 μCi of 3H-3-O-methyl-glucose was gavaged by oropharyngeal tube to conscious rats. Blood samples (10 μl) were collected 40 min after gavage and assayed for counts per minute (CPM) in plasma. To eliminate pain during tail vein sampling, 2% Lidocaine (0.1 ml) was injected 3-4 cm from the end of the tail (Gedulin, Jodka et al. Gastroenterology 108: A604, 1995).
Data Analysis:
[0062]Effects of the test compound were expressed as percent change relative to control, which was calculated as −100*(1-(mean value test rats / mean value controls)).
[0063]Relative activity was defined as significant if p<0.05 ...
example 3
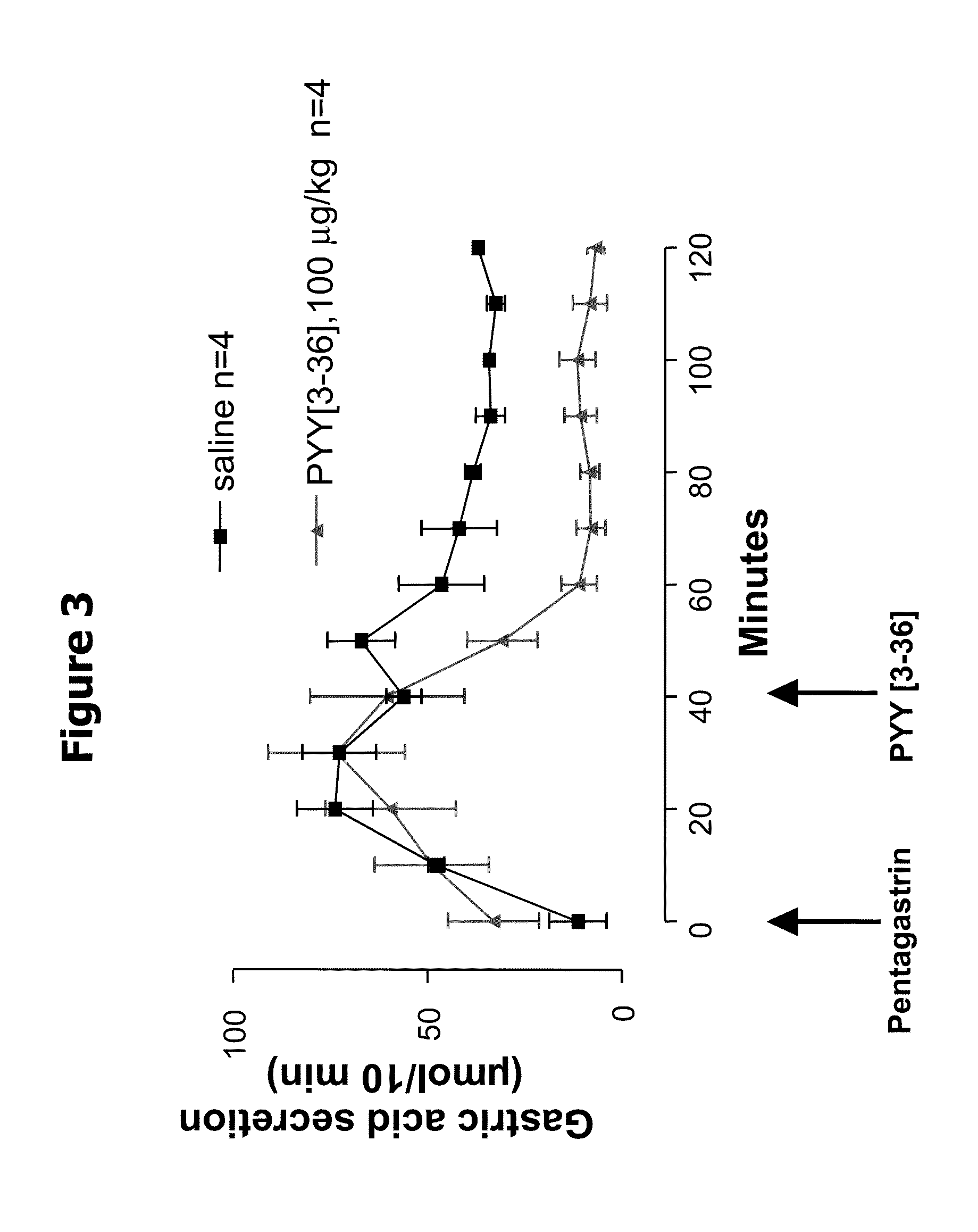
Acute Peripheral Administration of PYY[3-36] Inhibits Gastric Acid Secretion in Rats
[0065]Male Harlan Sprague Dawley rats were housed in a 12:12 hour light:dark cycle. All experiments were performed during the light cycle. Animals were fasted for approximately 20 hours before experimentation but were given free access to water until the start of the experiment.
[0066]Rats (age 11-16 weeks, body mass 291-365 g) were surgically fitted with gastric fistulae (Kato, Martinez et al. Peptides 16: 1257-1262, 1995). Overnight fasted rats were weighed and their gastric fistulae were uncapped and attached to flexible Tygon tubing (⅜× 1 / 16) into which was fitted a piece of PE205 tubing that would extend up into the stomach. Saline was injected through the narrower PE205 tubing and the effluent collected from the Tygon tubing. To ensure proper flow through the fistulae and an empty stomach, the stomach was flushed several times with ˜5 mL of room temperature saline solution until flow was easy an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| body mass | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com