Regulation of apg8 phosphorylation and uses thereof
a technology of apg8 and phosphorylation, applied in the direction of antibody medical ingredients, peptide/protein ingredients, immunological disorders, etc., can solve the problems of cell death, neuron dysfunction and neuron loss, and potentially unreliable markers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
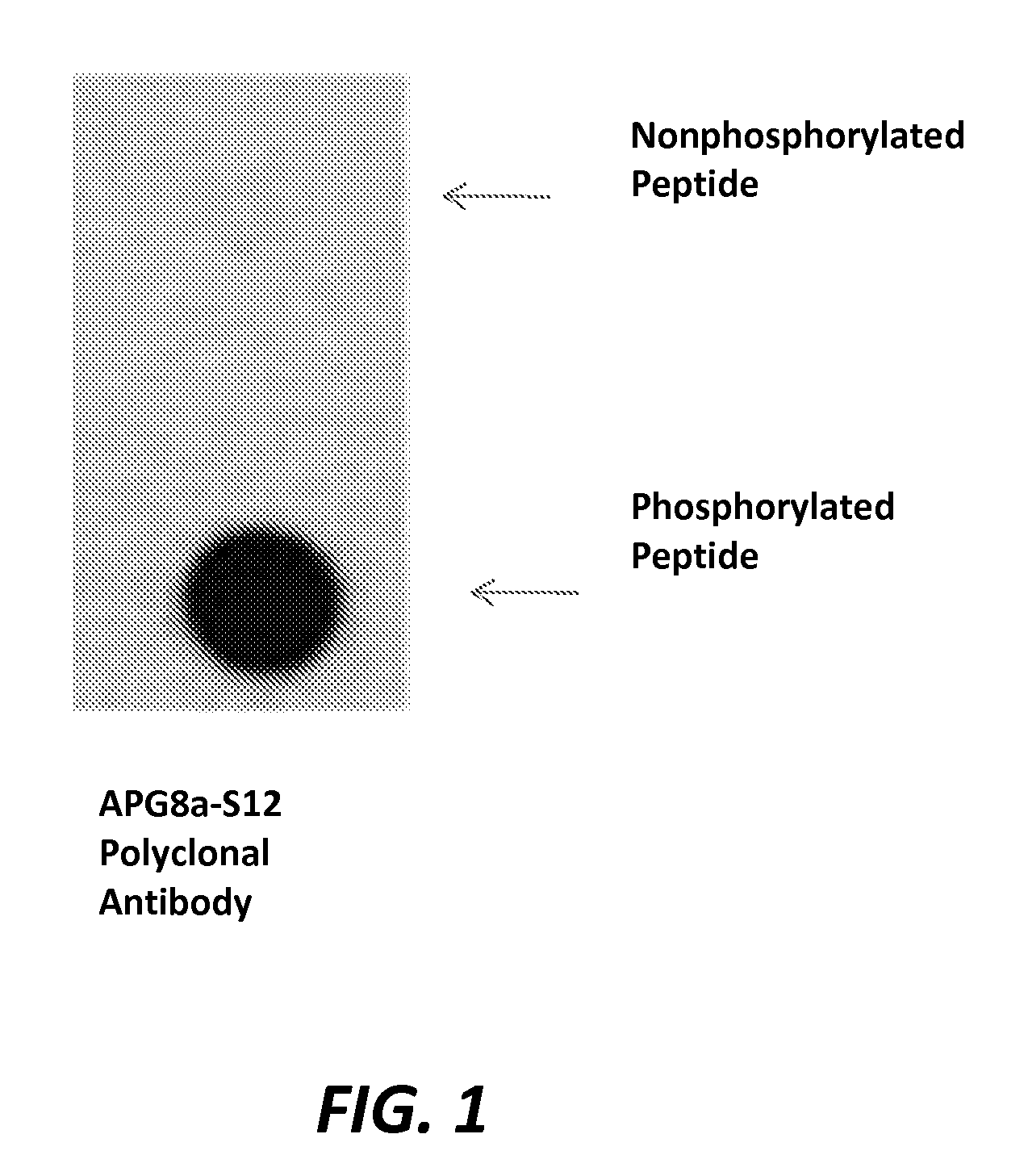
Production of an APG8a Phosphospecific Polyclonal Antibody
[0252]A 16 amino acid phospho-peptide antigen, MPSDRPFKQRRS*FADRC (SEQ ID NO:156) (where S*=phosphoserine), corresponding to residues 1-16 of human APG8a (SEQ ID NO:1) plus cysteine on the C-terminal for coupling, was constructed according to standard synthesis techniques using a Rainin / Protein Technologies, Inc., Symphony peptide synthesizer. See, e.g., ANTIBODIES: A LABORATORY MANUAL, Chapter 5, pages 75 76, Harlow & Lane Eds., Cold Spring Harbor Laboratory (1988); Czernik, Methods In Enzymology, 201: 264 283 (1991); Merrifield, J. Am. Chem. Soc. 85: 21-49 (1962)).
[0253]This peptide was coupled to KLH, and rabbits were injected intradermally (ID) on the back with antigen in complete Freunds adjuvant (200 μg antigen per rabbit). The rabbits were boosted with same antigen in incomplete Freund adjuvant (100 μg antigen per rabbit) weekly for eight. Coincident with the dates of the third through eighth boosts, 15-20 mL bleed per...
example 2
Western Blot Assay Using APG8a Antibody
[0255]The antibody described in Example 1 is tested for phosphospecificity in detection of cellular protein using Western blot assay. Jurkat and 293 cells are collected, washed with PBS and directly lysed in cell lysis buffer. The loading buffer is added into the respective cell lysate and the mixture is boiled at 100° C. for 5 minutes. 10-20 μg of lysate is added onto 7.5% SDS-PAGE gel. A standard Western blot is performed according to the Immunoblotting Protocol set out in the Abgent 2006-2008 Catalog and Technical Reference, p. 262. APG8a phosphospecific polyclonal antibody dilutions are optimized between 1:60 and 1:1000 from a stock concentration of 0.25 mg / mL. For each lysate, sample is incubated as follows: Lane 1, negative sera; Lane 2, phosphospecific APG8a antibody; Lane 3, phosphospecific APG8a antibody that is pre-incubated with phosphorylated peptide MPSDRPFKQRRS*FADRC (SEQ ID NO:156) (optimized in the range of 1:4 to 1:50 stoichiom...
example 3
Production of an APG8c Phosphospecific Polyclonal Antibody
[0256]A 16 amino acid phospho-peptide antigen, QKIPS*VRPFKQRC (SEQ ID NO:158) (where S*=phosphoserine), corresponding to residues 5-16 of human APG8c (SEQ ID NO:8) plus cysteine on the C-terminal for coupling, was constructed according to standard synthesis techniques using a Rainin / Protein Technologies, Inc., Symphony peptide synthesizer. See, e.g., ANTIBODIES: A LABORATORY MANUAL, Chapter 5, p. 75 76, Harlow & Lane Eds., Cold Spring Harbor Laboratory (1988); Czernik, Methods In Enzymology, 201: 264 283 (1991); Merrifield, J. Am. Chem. Soc. 85: 21 49 (1962)).
[0257]This peptide was coupled to KLH, and rabbits were injected intradermally (ID) on the back with antigen in complete Freunds adjuvant (200 μg antigen per rabbit). The rabbits were boosted with same antigen in incomplete Freund adjuvant (100 μg antigen per rabbit) weekly for eight. Coincident with the dates of the third through eighth boosts, 15-20 mL bleed per rabbit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap