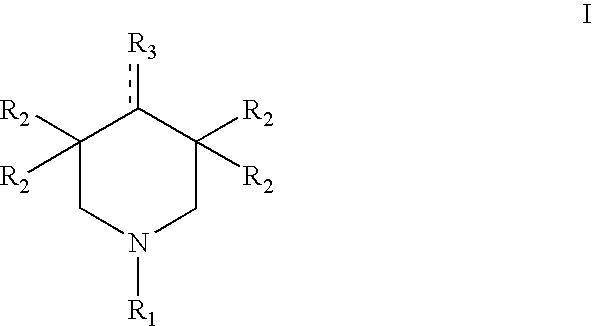
Marasmius androsaceus l.es fr extract, piperidone derivative, and their use for the preparation of antihypertensives
a technology of marasmius androsaceus and extract, which is applied in the field of marasmius androsaceus l. es fr extract and piperidone derivative, can solve the problems of serious threats to human health and lives, and achieve the effect of reducing blood pressur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of a Chloroform Extract
[0044]a. Culture of Seeds of Fungi Marasmius androsaceus L.es Fr:
[0045]A slant culture medium was prepared by mixing the following components (by weight): bran 1-10, glucose 0.3-3, peptone 0.2-2, magnesium sulfate 0.01-0.1, potassium dihydrogen sulfate 0.02-0.2, agar 0.5-5 and water 100. Then, a strain was inoculated to the slant plane and cultured at 20-30° C. for 10-20 days.
[0046]b. Fermentation Culture of Fungi Marasmius androsaceus L.es Fr:
[0047]A culture medium was prepared by mixing the following components (by weight): bran 3-30%, glucose 1-10%, corn slurry liquid 0.2-20%, magnesium sulfate 0.01-8%, and potassium dihydrogen sulfate 0.05-9%. The slant strain is transferred to the fermentation culture medium, and cultured by fermentation at 20-30° C. for 5-10 days until forming sericate hypha, and the culture medium becoming faint yellow. The culture was stopped at pH=1.5-6.0, and then the fermentation broth is allowed to stand by for 5-10 day...
example 2
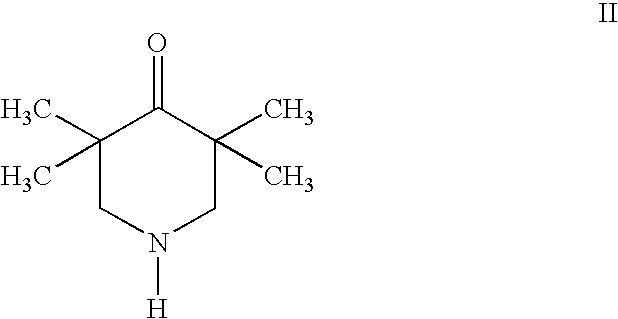
Preparation of a compound of formula II (3,3,5,5-tetramethyl-4-piperidone)
[0050]The chloroform extract obtained in step c of Example 1 was separated with a silica gel column using chloroform:methanol:ammonia water=9:1:0.1 as developing agent. The eluents containing the compound of formula II were combined and concentrated to obtain a compound monomer of formula II.
[0051]The compound of formula II was a white needle crystal, with a melting point of 54-57° C. (decomposition).
Elemental analysis: C9H17NOC(%)H(%)O(%)Analysis values69.4810.9610.12Calculated values69.6810.9710.32
[0052]MS (+FAB)m / z: 156.2, 149.2, 102.2, 98.2, 83.1, 74.0, by which the compound was verified as having a molecular weight of 155.
[0053]IR(KBr)cm−1: 3318.93, 2910.09, 2755.81, 1727.93, 1626.67, 1726, 170, 1727.93, 1626.67, 1726.23, 2317.05, 2997-2465.
UV λMeOHMAXnm: 264.3 (ε13256).
[0054]1H-NMR (DMSO, TMS) δ ppm 1.48 (S, 12H,(CH3)4), 2.63 (S, 4H,(CH2)2) 9.67 (S, 1H, NH).
[0055]13C-NMR (DMSO-D6,TMS) δ ppm: 27.20 (CH3)4...
example 3
Preparation of 1-ethyl-3,3,5,5-tetramethyl-4-piperidone (Compound of Formula Ia)
[0057]0.3 g (1.94 mmol) of the compound of formula II obtained in Example 2 and 7.5 mmol of bromoethane were dissolved with 40 mol of anhydrous ethanol. The solution was charged to a 100 ml three-neck flask equipped with a reflux condenser, a stirrer, an internal thermometer and a dropping funnel. An ethanol solution containing 8.5 mmol sodium ethoxide was added to the flask with stirring followed by reacting the system at 50° C. for 20-50 min. After cooling down, 20 ml of chloroform was dropped to the system before standing by for a certain time. The resultant product was filtered to remove sodium bromide, and the filtrate was concentrated to dry under vacuum condition. Then, the reaction product was separated with a silica gel column, and eluted using chloroform:methanol (5:1), thereby obtaining the titled compound of formula Ia: 1-ethyl-3,3,5,5-tetramethyl-4-piperidone.
[0058]FAB-MS m / z: 185[M+H]+, by ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com