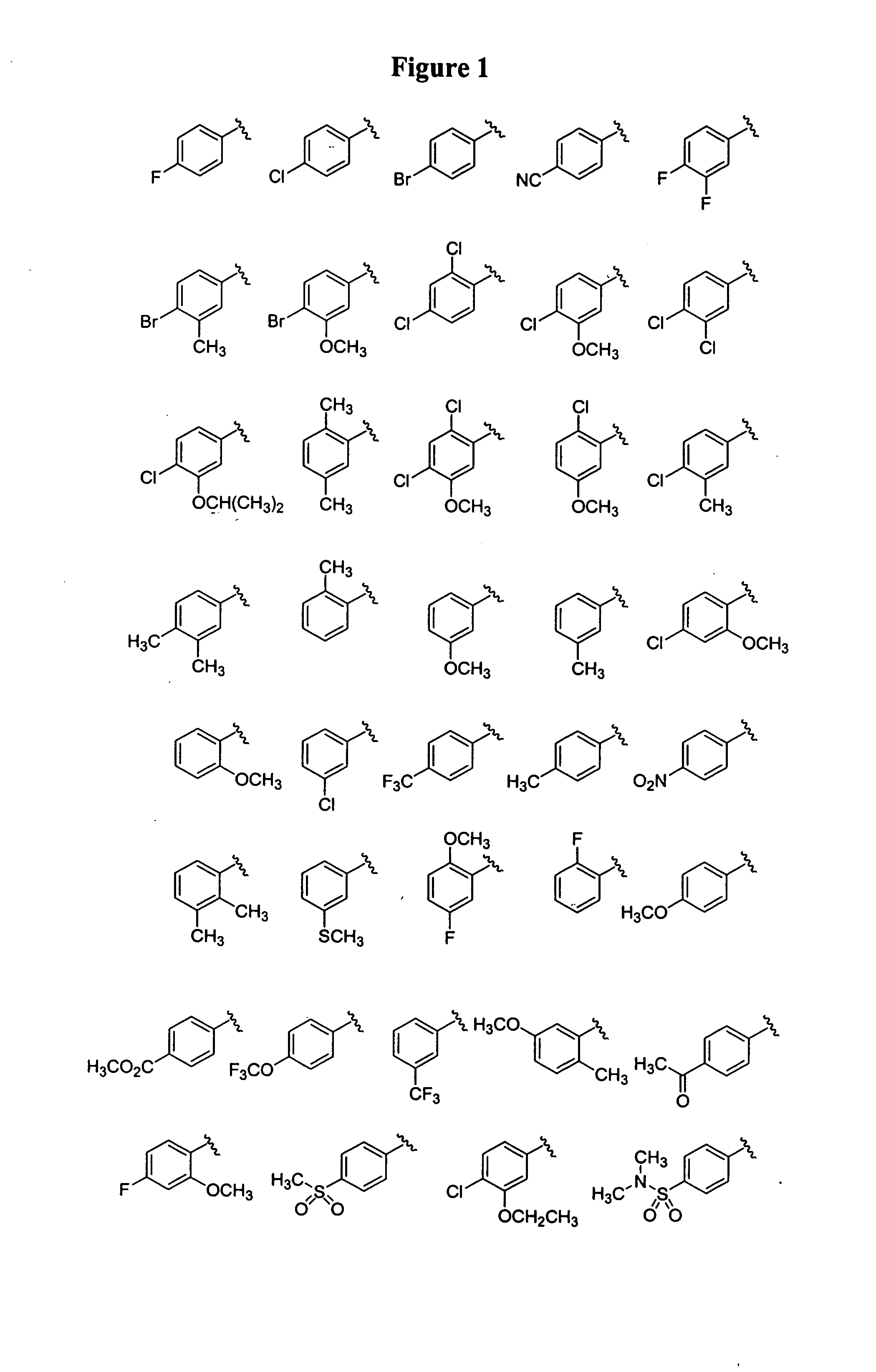
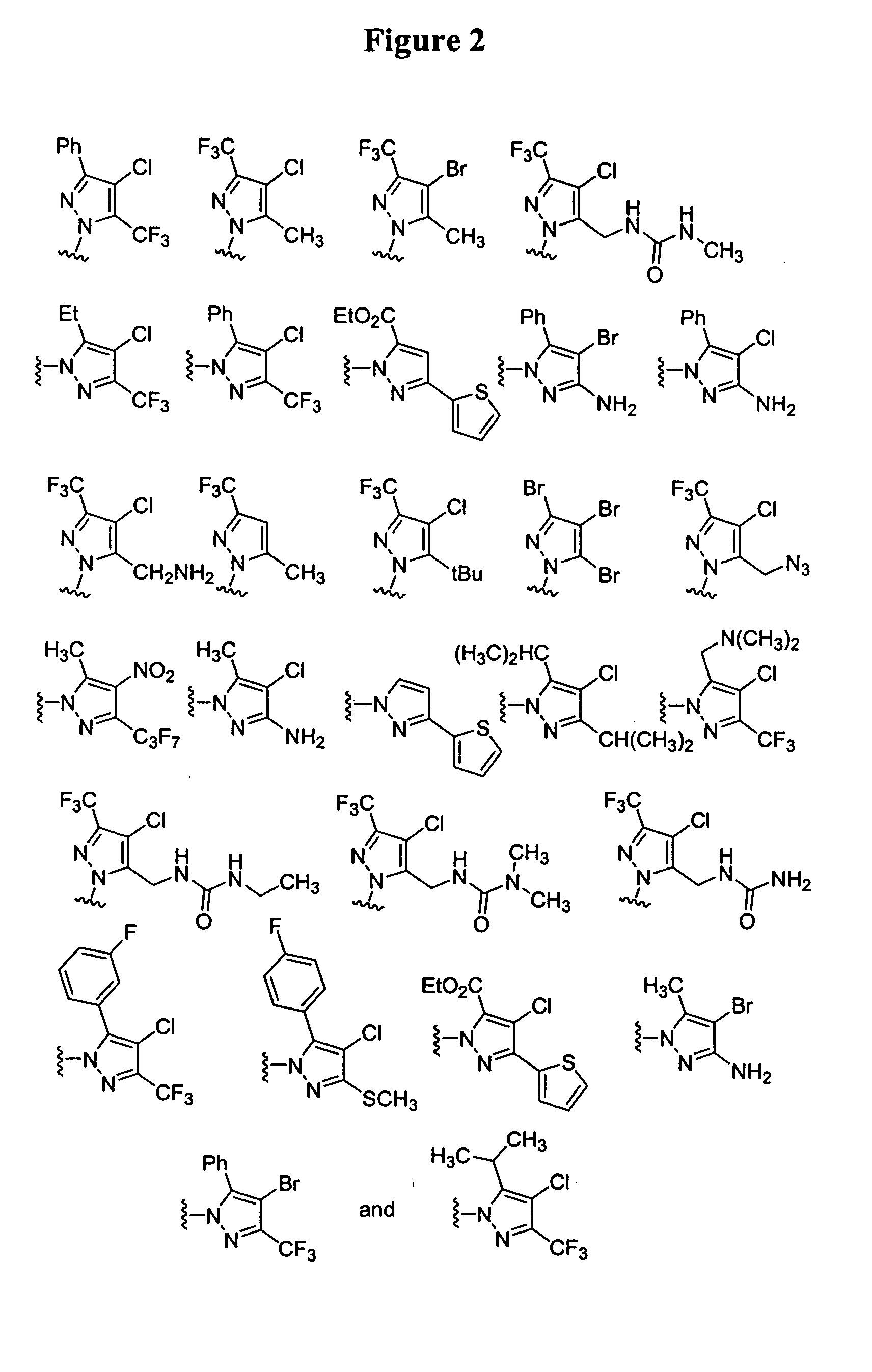
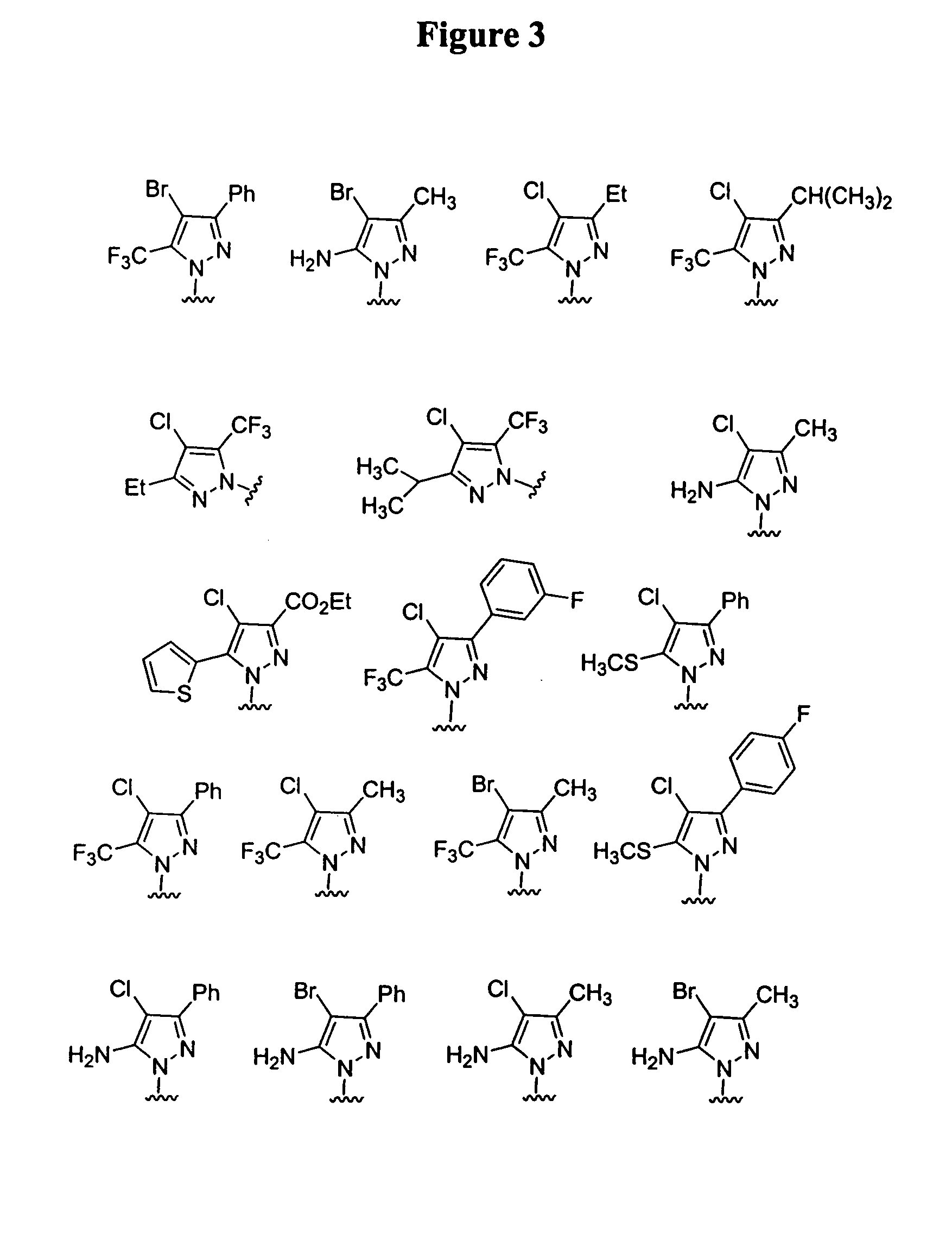
Substituted piperazines
a technology of substituted piperazines and substituted piperazines, which is applied in the direction of biocide, drug composition, immunological disorders, etc., can solve the problems of inability to fully absorb the drug, short half-lives once administered, and prohibitive development and manufacturing costs, so as to achieve favorable solubility in water and increase the partitioning into selected solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0112]The piperazine ring can be formally attached to the terminal aryl unit in a number of ways: by aromatic nucleophilic displacement reactions, metal catalyzed coupling reactions (arylation reactions of secondary amines), ring expansion, rearrangement and cyclization reactions and the like. Also, different protection / deprotection strategies can be utilized. Hence, either all or only part of the final molecular architecture can be present during the key aryl coupling step. Examples for a variety of such aryl coupling strategies are listed below.
Protocol A
Metal Catalysed Arylation Reactions of Secondary Amines
Synthesis of (5-Chloro-2-piperazin-1-yl-phenyl)-phenyl-methanone
[0113]
[0114]Piperazine (3.6 g, 42.5 mmol), Pd(II)acetate (0.007 g, 0.043 mmol), sodium t-butoxide (0.22 g, 2.4 mmol) and BINAP (0.042 g, 0.068 mmol) were stirred at room temperature in 10 mL dry toluene for 15 min. (2-Bromo-5-chloro-phenyl)-phenyl-methanone (0.5 g, 1.7 mmol) in 10 mL dry toluene was then added int...
example 2
[0569]This example illustrates the activity associated with representative compounds of the invention.
[0570]Materials and Methods
A. Cells
[0571]CCR1 Expressing Cells
[0572]a. THP-1 Cells
[0573]THP-1 cells were obtained from ATCC and cultured as a suspension in RPMI-1640 medium supplemented with 2 mM L-glutamine, 1.5 g / L sodium bicarbonate, 4.5 g / L glucose, 10 mM HEPES, 1 mM sodium pyruvate, 0.05% 2-mercaptoethanol and 10% FBS. Cells were grown under 5% CO2 / 95% air, 100% humidity at 37° C. and subcultured twice weekly at 1:5 and harvested at 1×106 cells / ml. THP-1 cells express CCR1 and can be used in CCR1 binding and functional assays.
[0574]b. Isolated Human Monocytes
[0575]Monocytes were isolated from human buffy coats using the Miltenyi bead isolation system (Miltenyi, Auburn, Calif.). Briefly, following a Ficoll gradient separation to isolate peripheral blood mononuclear cells, cells were washed with PBS and the red blood cells lysed using standard procedures. Remaining cells were lab...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mol % | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com