Transgenic animal
a technology of transgenic animals and knockouts, applied in the field of transgenic animals, can solve the problems that the phenotypic effect of a simple knockout fails to provide useful or accurate information about the function of the gene, and achieve the effect of modulating gpcr signalling and inhibiting rgs activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cloning of the Transgene PCR
Amplification and Cloning of The Gαq G188S Transgene
[0078]Plasmid pcDNAamp Gqsst (DiBello et al. J. Biol. Chem. 1998, 273:5780-5784) was used as a template to PCR a 1.1 kb fragment of the mouse Gαq cDNA. Gqsst contains a G to A conversion at nucleotide 573 and a G to C conversion at nucleotide 575 that converts a glycine residue to serine at amino acid residue 188 (G188S) (see FIG. 1). In addition, several nucleotide changes were incorporated 5′ to the mutation to convert an endogenous Gαq amino acid sequence portion to that of an EE epitope tag (FIG. 1) to allow mutant Gαq protein detection using anti-EE antisera. “EE” refers to a two glutamate sequence that is part of an epitope recognized by a commercially available mouse monoclonal antibody Glu-Glu (Babco, Berkley Antibody Company, Richmond, Calif.). This antibody was raised against the sequence CEEEEYMPE and is specific for either six amino acid sequences EYMPME or EFMPME. The EE epitope is known to ...
example 2
Construction of Tissue Specific Transgene
Expression Construct Cloning of Thy1.2-Gαq (G188S)
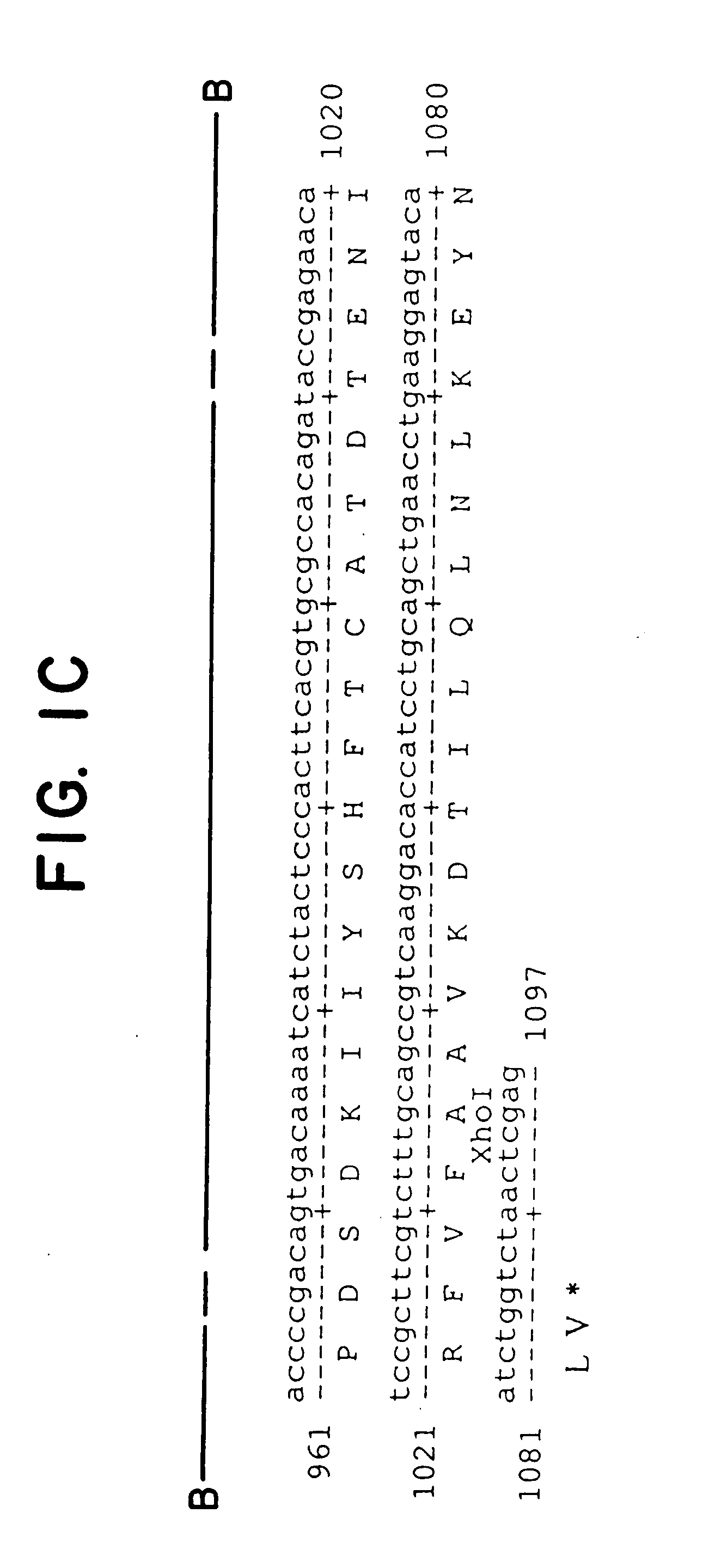
[0079]Plasmid Gαq (G188S)-GemT was cut with Xho I and the 1.1 kb Gαq cDNA was excised from a 1% agarose gel and cluted by Gene-Clean (Bio-101) according to manufacturer's protocol. Plasmid Thy1.2 containing a 6.7 kb NotI-PvuI mouse Thy1.2 minigene cloned in pUC19 was described previously. The 1.1 kb Gαq DNA was ligated to Thy1.2 vector DNA cut with Xho I and treated with calf intestinal alkaline phosphatase to remove 5′ phosphate ends (Sambrook et al., 1982). Clone Thy1.2-Gαq (G188S) containing the 1.1 kb mutant Gαq cDNA in the correct orientation in the XhoI cloning site of the Thy1.2 expression cassette was verified by restriction mapping as well as DNA sequence analysis (see FIG. 2).
example 3
Preparation of Transgenic Rat
Transgene Preparation For DNA Microinjection
[0080]Construct Thy1.2-Gαq G188S DNA (100 micrograms) was cut with NotI and PvuI restriction enzymes overnight at 37° C. The DNA was electrophoresed on 1% low melting point agarose gel (BRL) to allow separation of the 7.8 kb Thy1.2-Gαq G188S transgene DNA from smaller sized 2 kb and 1 kb fragments generated from restriction of the pUC19 vector (New England Biolabs) backbone. The 7.8 kb fragment was excised from the LMP gel and heated to 70° C. to melt the agarose. Once liquefied, an equal volume of phenol was used to extract the gel mix. The aqueous portion was re-extracted using a phenol-chloroform mix followed by chloroform. The aqueous fraction was then precipitated using 2 volumes of 95% ethanol, followed by a 70% ethanol wash. The DNA pellet was resuspended in 2.5 ml TE buffer. One gram of cesium chloride (Sigma, St. Louis, Mo.) was added for every 1 ml of DNA-TE mix and gently dissolved. Ethidium bromide ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com