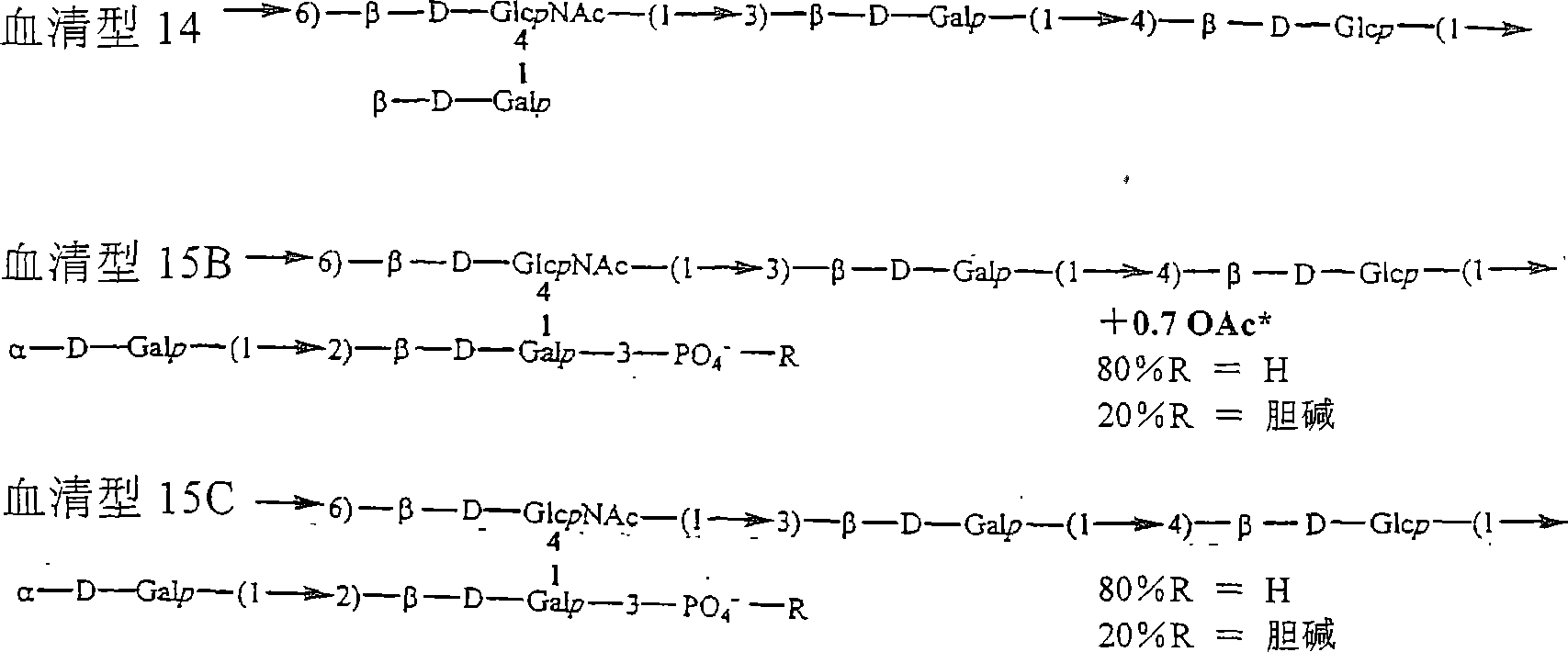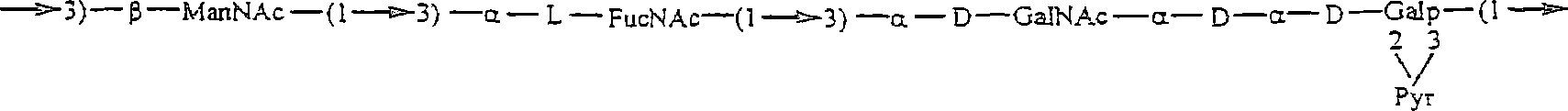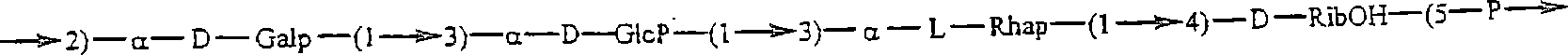Induction of an immune response against streptococcus pneumoniae polysaccharides
A Streptococcus pneumoniae, polysaccharide technology, applied in bacterial peptides, antibacterial drugs, bacterial antigen components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0237] 3. Preparation of Conjugates
[0238] Conjugation of the desired polysaccharide to a polypeptide comprising the pan DR peptide sequence can be accomplished by a number of methods. Polysaccharides can be attached to the polysaccharides of the invention by enzymatic or chemical reactions. A wide range of ligation strategies are described, for example, in Hermanson, BIOCONJUGATE TECHNIQUES (Academic Press, 1996); Lockhart, "Conjugate Vaccines," Expert Rev. Vaccines 2(5):633-648 (2003). Ionic interactions are possible via the termini or via the ε-amino groups of lysines. Hydrogen bonding between residue side groups and epitopes is also possible.
[0239] In some embodiments, the polysaccharide / pan DR binding peptide conjugates are linked by a spacer or linker. Alternatively, the polysaccharide can be directly attached to the pan DR binding peptide without a linker.
[0240] The spacers or linkers may consist of neutral molecules, such as aliphatic carbon chains, amino a...
Embodiment
[0278] The following examples are offered by way of illustration and not limitation.
[0279] An experimental carbohydrate-conjugate vaccine was produced consisting of a 13 amino acid universal PanHLA-DR epitope and S. pneumoniae envelope polysaccharides from serotypes 14, 6B and 9V. Simple carbodiimide-mediated condensation chemistry was used to conjugate the pan DR-bound synthetic peptides to 3 chemically distinct capsular polysaccharides in a 1:1 molar ratio. The immunogenicity of the pan DR-binding peptide component of the conjugate vaccine was demonstrated by inducing a pan DR-binding peptide-specific CD4+ helper T cell (HTL) response after immunization of C57BL / 6 mice. Apply complete Freund's adjuvant and aluminum glue Al(OH) 3 formulation, induces high-titer antibody responses specific to Streptococcus pneumoniae serotypes 14, 6B and 9V. The effect of the HTL, or vector, pan DR binding synthetic peptide was only evident when using the pan DR binding peptide-polysaccha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com