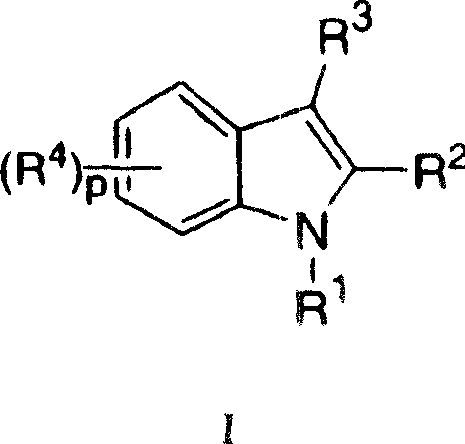
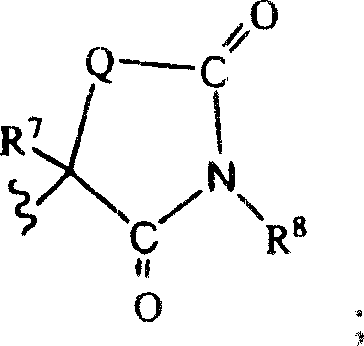
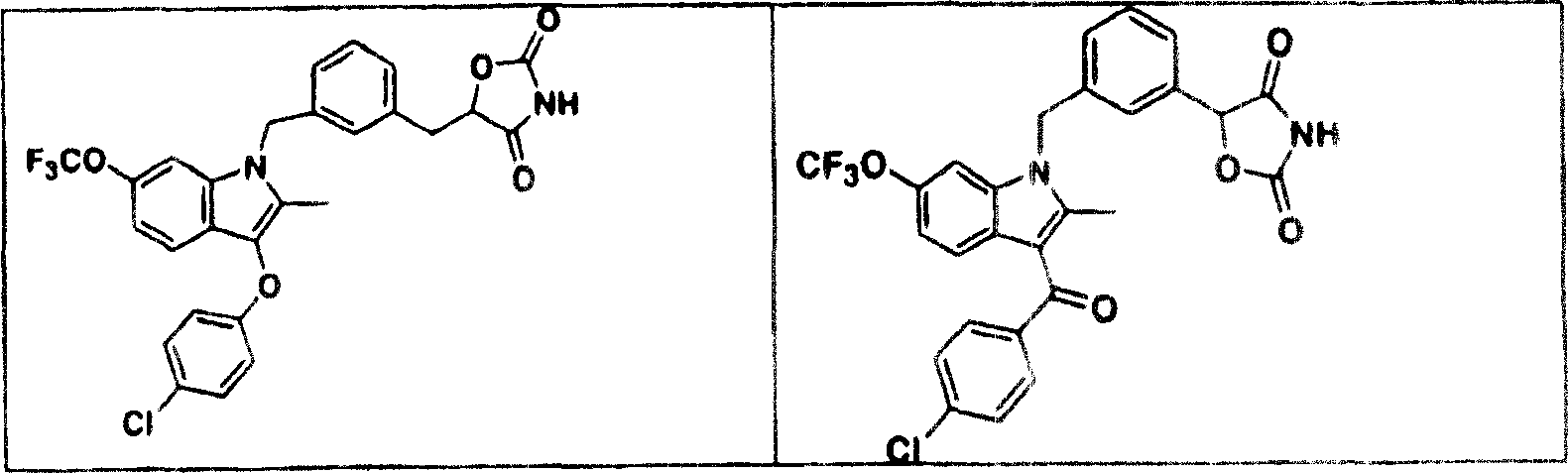
Indoles having anti-diabetic activity
一种选自、化合物的技术,应用在有机活性成分、含有效成分的医用配制品、性疾病等方向,能够解决脂类负面影响、不能显著改善脂类代谢等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0265] Embodiment 1 (see scheme 5)
[0266] Step 1: Preparation of Intermediate 4
[0267]
[0268] To a solution of 2-glycolamide (14 g, 186 mmol) and diethyl carbonate (27.1 mL, 224 mmol) in methanol (200 mL) was added potassium tert-butoxide (20.8 g, 186 mmol). The reaction mixture was refluxed overnight and then cooled to room temperature. Solvent was removed in vacuo. The residue was dissolved in brine, acidified with 2N hydrochloric acid, and extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate, filtered and concentrated in vacuo to afford 8.0 g of oxazolidinedione as a white solid.
[0269] To a solution of oxazolidinedione (4.6 g, 45 mmol) obtained above in dichloromethane (50 mL) was added TrCl (12.6 g, 45 mmol) and Et 3 N (6.3 mL, 45 mmol). The reaction mixture was stirred at room temperature for 40 minutes, then partitioned between water and ethyl acetate. The organic layer was washed with water and brine, dried...
Embodiment 2
[0277] Embodiment 2 (see scheme 6)
[0278] Step 1: Preparation of intermediate 13
[0279]
[0280] To a solution of sodium bisulfate (10.7 g, 103 mmol) in water (150 mL) was added aldehyde 8 (19.3 g, 86 mmol) at 50 °C. The reaction was stirred at 50°C for 1.5 hours, then cooled to 0°C. Join Et 2 O (100 mL), then a suspension of NaCN (4.66 g) in water (100 mL) was added. After 2 hours at 0°C the layers were separated. The aqueous layer was extracted with ether. The combined organic layers were washed with brine, dried over sodium sulfate, filtered and concentrated in vacuo to give the product cyanohydrin as an oil.
[0281] To a solution of the product obtained above (10 g) in formic acid (30 ml) was slowly added concentrated hydrochloric acid (30 ml) at 0°C. The reaction was stirred at 0°C for 2 hours, poured onto crushed ice, and extracted with ethyl acetate (2x). The combined organic layers were washed with water, 1.0 N aqueous sodium hydroxide solution (3x), b...
Embodiment 3
[0289] Embodiment 3 (see scheme 7)
[0290] Step 1: Preparation of (R)-diol 18
[0291]
[0292] To a suspension of methyltriphenylphosphine bromide (33.2 g, 92.9 mmol) in diethyl ether (200 mL) was slowly added n-butyllithium (2.5M in hexane, 37 mL) at room temperature. The resulting yellow solution was stirred for 30 minutes before the addition of ketone 16 (12 mL, 90 mmol). The reaction was stirred overnight at room temperature. The precipitate was removed by filtration. Solvent was removed in vacuo. Purification by flash chromatography afforded the alkene product.
[0293] To a suspension of AD-mixture-β (7 g) in water / tert-butanol (25 mL / 25 mL) was added the olefin (0.66 g, 5 mmol) obtained above at 0°C. The reaction mixture was stirred overnight at 0 °C. Sodium sulfite (7.5 g) was added. After 1 hour at room temperature, the mixture was extracted with ethyl acetate (3x). The combined organic layers were washed with brine, dried over sodium sulfate, filtered ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com