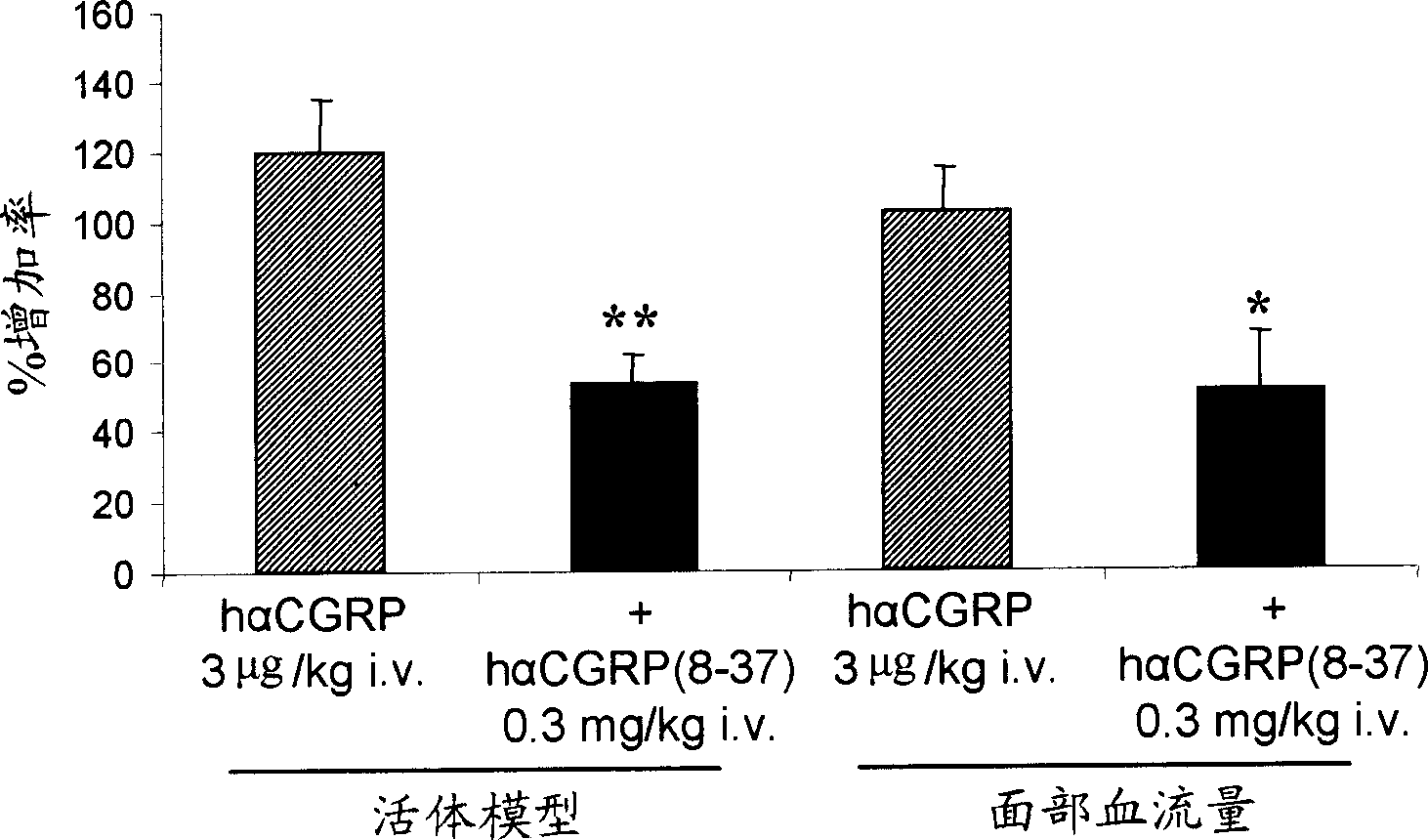
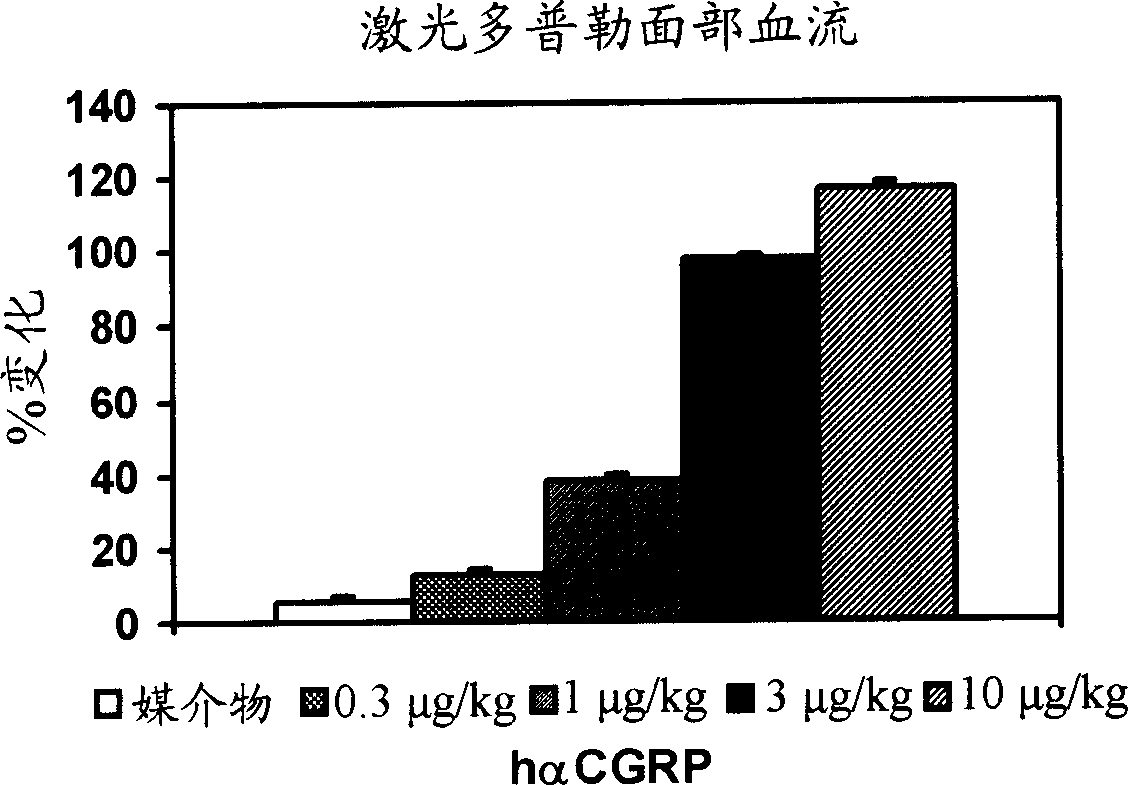
Calcitonin gene related peptide receptor antagonists
A methyl and cyano technology, applied in the field of calcitonin gene-related peptide receptor antagonists, can solve the problem of not showing the effect of arterial dilatation or increased blood flow interruption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0293] HNR 1 R 2 and the preparation of formula VIII amine
[0294] Formula VIII and HNR 1 R 2 Amines are either commercially available or can be prepared by literature methods or methods described therein.
[0295]
[0296] Preparation of Amino Acids of Formula II and V
[0297]
[0298] Amino acids of Formula II and V are either commercially available or can be prepared as described in Scheme 4.
[0299] Scheme 4. Synthesis of Formula II and Formula V Compounds
[0300]
[0301] The synthesis described in Scheme 4 begins with an aldehyde of formula IX which is reacted with a glycine phosphonate of formula X via Wadsworth-Emmons coupling. Compounds of formula X are deprotonated with a base such as diazabicycloundecene or tetramethylguanidine or other organic or inorganic bases well known in the art. Reduction of the double bond of the resulting compound of formula XI affords the compound of formula XII. This reduction is carried out to give the racemate, or b...
Embodiment 1
[0447] (±)-3-(1H-indazol-5-yl)-2-{[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine- 1-Carbonyl]-amino}-propionic acid
[0448]
[0449] 5-(2-Methoxycarbonyl-2-{[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piper A solution of pyridine-1-carbonyl]-amino}-ethyl)-indazole-1-carboxylic acid tert-butyl ester (168 mg, 0.29 mmol) was cooled to 0 °C in methanol (5 mL). An aqueous solution (5 mL) of lithium hydroxide monohydrate (49 mg, 2.04 mmol) was added. The reaction mixture was stirred at 0°C for 6 hours and then placed in the freezer for an additional 16 hours. The solvent was removed in vacuo, and the residue was dissolved in water (15 mL). The pH of the aqueous solution was adjusted to about 1 with 1N hydrochloric acid. The precipitated white solid was collected by filtration. The solid was dried in vacuo to give the title compound (108 mg, 80%).
[0450] 1 H-NMR (DMSO-d 6 , 300MHz) δ12.94(bs, 1H), 9.19(s, 1H), 8.01(s, 1H), 7.61(s, 1H), 7.46(d, J=8.4Hz, 1H), 7.28(dd,...
Embodiment 2
[0495] (R)-4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-[1,4′]bipiperidine- 1'-yl-1-(1H-indazol-5-ylmethyl)-2-oxo-ethyl]-amide ()
[0496]
[0497] (R)-4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid {2-[1,4′]bipiperidine -1'-yl-2-oxo-1-[1-(2-trimethylsilyl-ethanesulfonyl)-1H-indazol-5-ylmethyl]-ethyl}-amide (568 mg, 0.73 mmol) and cesium fluoride (1.11 g, 7.31 mmol) in acetonitrile (50 mL) was heated at 80°C for 4.5 hours. The reaction mixture was concentrated, and the residue was subjected to flash column chromatography (dichloromethane / methanol / triethylamine, 94:5:1) to afford 280 mg (63% yield) of the title compound as a white solid, which was recovered by using Chirocel HPLC analysis of the OD column determined to have 98.2% ee with 20% B (A = ethanol, B = 0.05% diethylamine in hexane) as eluent (retention time: 9.51 min for the title compound, and 9.51 min for the S- Enantiomer 15.9 minutes). 1 H-NMR (CD 3 OD, 500...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com