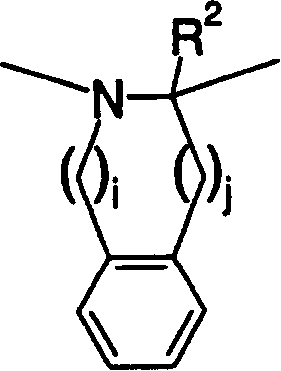
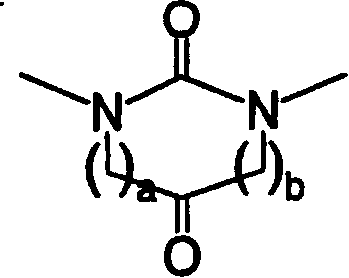
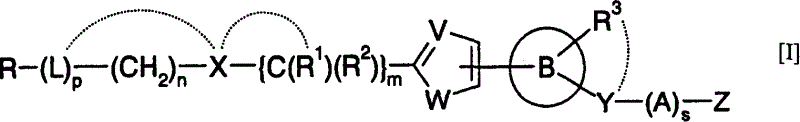Heteroaromatic pentacyclic compound and medicinal use thereof
A technology of heteroaromatic rings and compounds, applied in the field of new 5-membered heteroaromatic ring compounds, which can solve the problems of no description, no disclosure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[1702] 5-{4-[4-({[4-(1-Ethylpropyl)phenyl]isopropylamino}methyl)-phenyl]thiazol-2-ylmethoxy}nicotinic acid
[1703] (1) 4-(2-(Benzoyloxymethyl)thiazol-4-yl)benzoic acid
[1704]
[1705] To a solution of 4-(bromoacetyl)benzoic acid (10 g, 41.14 mmol) in N,N-dimethylformamide (40 ml) was added 2-(benzoyloxy)ethylthioamide ( 8.03g, 41.14mmol), and the mixture was stirred at room temperature for 1.5 hours. After completing the reaction, sodium bicarbonate (3.46 g, 41.14 mmol) and water were added under ice-cooling, the mixture was stirred at room temperature for 0.5 hour, and the precipitate was collected by filtration. The obtained solid was reduced under reduced pressure to provide the title compound (13.089 g, 93.3%).
[1706] (2) (4-(4-hydroxymethylphenyl)thiazol-2-yl)methyl benzoate
[1707]
[1708] To 4-(2-(benzoyloxymethyl)thiazol-4-yl)benzoic acid (0.5g, 1.47mmol) obtained in Example 1-1(1) in tetrahydrofuran (5ml) at room temperature 1,1'-Carbondiimidazole (0....
Embodiment 1-2 to 1-186
[1727] In the same manner as in Example 1-1, using other conventional methods when necessary, the compounds of Examples 1-2 to 1-186 were produced. Structural formulas and property values of the obtained compounds and those in Example 1-1 are shown in the following table.
[1728]
[1729]
[1730]
[1731]
[1732]
[1733]
[1734]
[1735]
[1736]
[1737]
[1738]
[1739]
[1740]
[1741]
[1742]
[1743]
[1744]
[1745]
[1746]
[1747]
[1748]
[1749]
[1750]
[1751]
[1752]
[1753]
[1754]
[1755]
[1756]
[1757]
[1758]
[1759]
[1760]
[1761]
[1762]
[1763]
[1764]
[1765]
[1766]
[1767]
[1768]
[1769]
[1770]
[1771]
[1772]
[1773]
[1774]
[1775]
[1776]
[1777]
[1778]
[1779]
[1780]
Embodiment 2-1
[1782] {Benzyl-[4-(4-{methyl[4-(1-propylbutyl)benzyl]amino}-phenyl)oxazol-2-ylmethyl]amino}acetic acid
[1783] (1) 4-(1-Propylbutyl)benzaldehyde
[1784]
[1785] To a solution of titanium tetrachloride (18.7ml, 170mmol) in chloroform (50ml) was added dropwise (1-propylbutyl)benzene (10.0g, 56.7mmol) and dichloromethyl methyl ether (7.70mmol) under ice-cooling. ml, 85.1 mmol) in chloroform (40 ml), and the mixture was stirred at the same temperature for 1 hour. The reaction mixture was poured into ice (100 g) and the mixture was stirred at room temperature for 1 hour. The organic layer was successively washed with water, 0.5N aqueous sodium hydroxide solution, water and saturated brine, and dried over magnesium sulfate. After filtration, the solvent was removed and the residue was purified by silica gel column chromatography (eluent; ethyl acetate-hexane 1:50) to provide the title compound (10.0 g, yield 87%).
[1786] (2) N-methyl-4-(1-propylbutyl)benzylamine hydrochlo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap