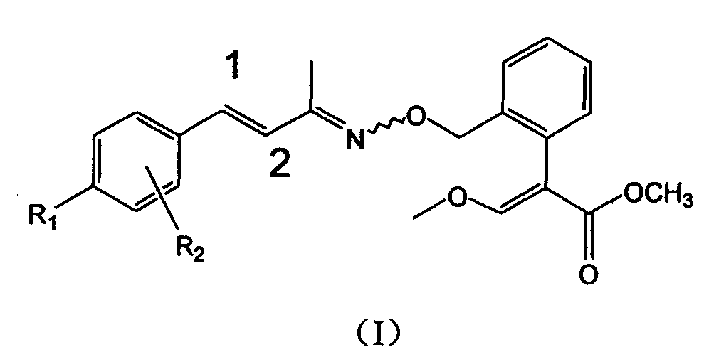
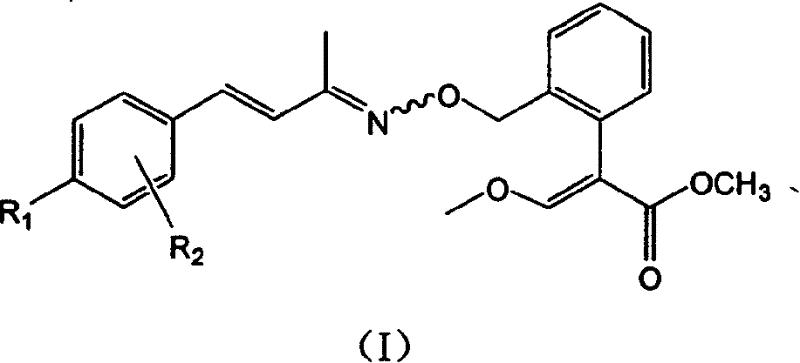
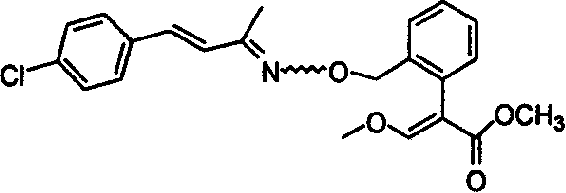
Unsaturated oximino ether compound with fungicidal, insecticidal activity
A compound, unsaturated technology, applied in the field of pesticides and agricultural fungicides, can solve the problem of no further research and achieve high fungicidal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0043] Synthesis of compounds in Example 1 Table 1:
[0044] 14 grams of p-chlorobenzaldehyde was dissolved in 30 milliliters of acetone, 5 grams of 10% sodium hydroxide solution was added dropwise thereto, the temperature was maintained not higher than 25° C., and stirring was continued for 1 hour after the addition was completed. The mixture was poured into water, extracted with ethyl acetate, washed with water three times, then dried and concentrated to give 15.5 g of light yellow oily 4-chlorostyryl methyl ketone, with a yield of 86%.
[0045] 54 g of 4-chlorostyryl methyl ketone, 52 g of hydroxylamine hydrochloride and 30 g of sodium hydroxide were refluxed in 1000 ml of methanol for 2 hours, then the reaction solution was concentrated, diluted with water and extracted with ethyl acetate. The combined extracts were dried and concentrated to obtain 32 g of 4-chlorostyryl methyl ketone oxime as an oil.
[0046] A solution of 9.5 g of 4-chlorostyrylmethylketoxime in DMF (10...
example 2
[0047] The separation of compound in example 2 table 1
[0048] The above oil was subjected to column chromatography with a mixture of ethyl acetate and petroleum ether (1:10) as the eluent, and the mixture of the above oil with ethyl acetate and petroleum ether (1:10) was collected separately. Column chromatography was carried out for the eluent, and each component was collected separately. The Rf values were 0.3 (compound 1), 0.28 (compound 2), 0.23 (compound 3), and 0.21 (compound 4), and four compounds were obtained.
[0049] Biotest example
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com