Method and device for modulating the immune response
A technology of immune response and immune cells, which is applied in the field of regulating the immune response to antigens, and can solve problems such as treatment technology and material difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
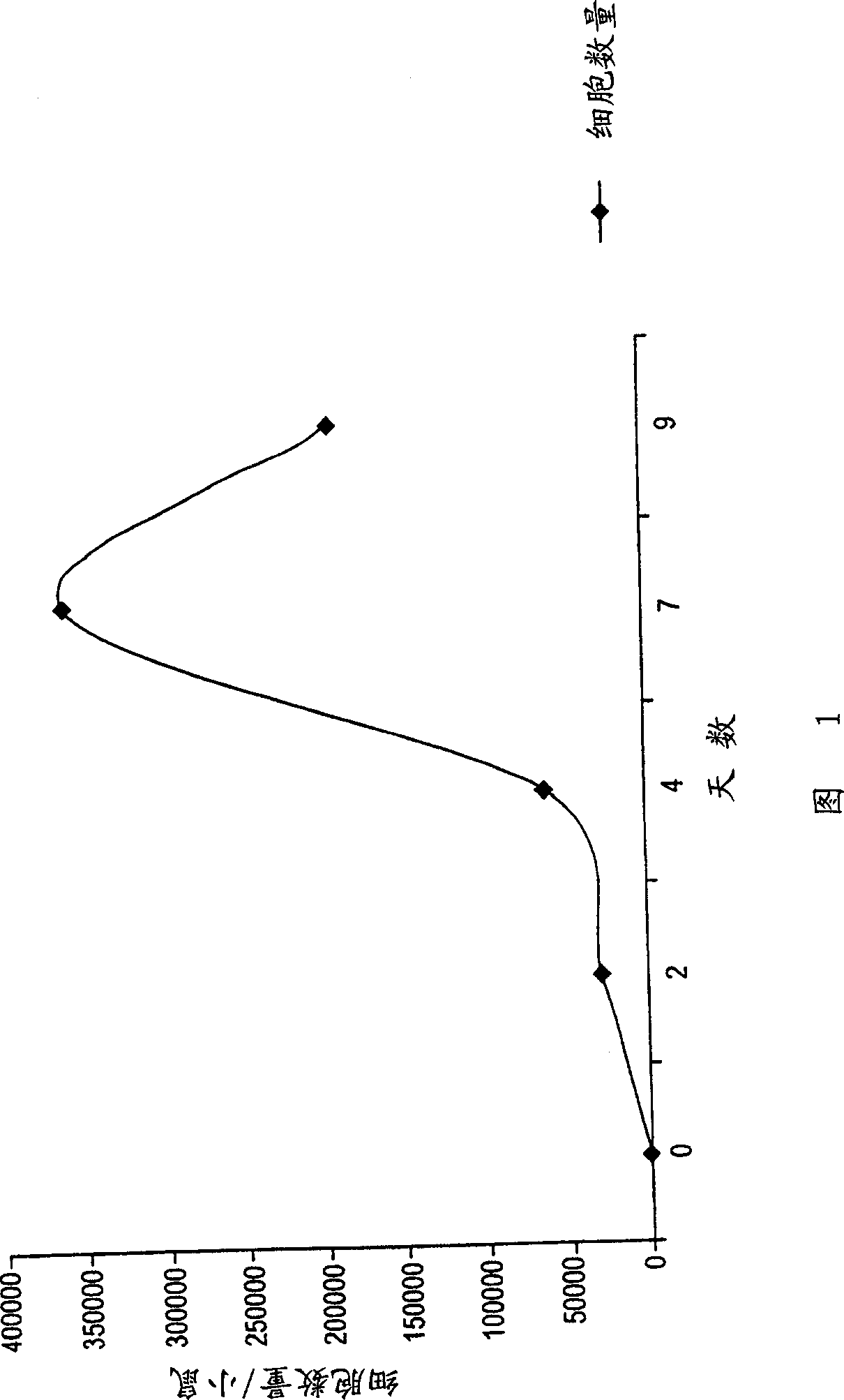
[0073] An example of making the device of the present invention is to take a piece of silicone tubing with an inner diameter of 0.15 cm, an outer diameter of 0.2 cm, and a length of 2.5 cm, and fill it with a length of hydroxylated polyvinyl acetate sponge of 2.5 cm. The device was submerged in a container containing phosphate buffered saline and autoclaved. Anesthetize female BALB / c mice (6-8 weeks old) with tribromoethanol. The device was inserted through a 0.5 cm incision in the dorsal midline on day 1. At 2 days post-implantation, some animals were introduced into the device with 50 μl of 1 mg / ml influenza antigen (FLUSHIELD® influenza virus vaccine, trivalent, types A & B; from Henry Schein®, Melville NY) by piercing the device with an injection needle. The skin near the implanted device is penetrated, and the needle is then guided into one end of the device by feel.
[0074] Fluid was aspirated from the implanted devices of 5 animals each at 2, 4, 7 and 9 days post-imp...
Embodiment 2
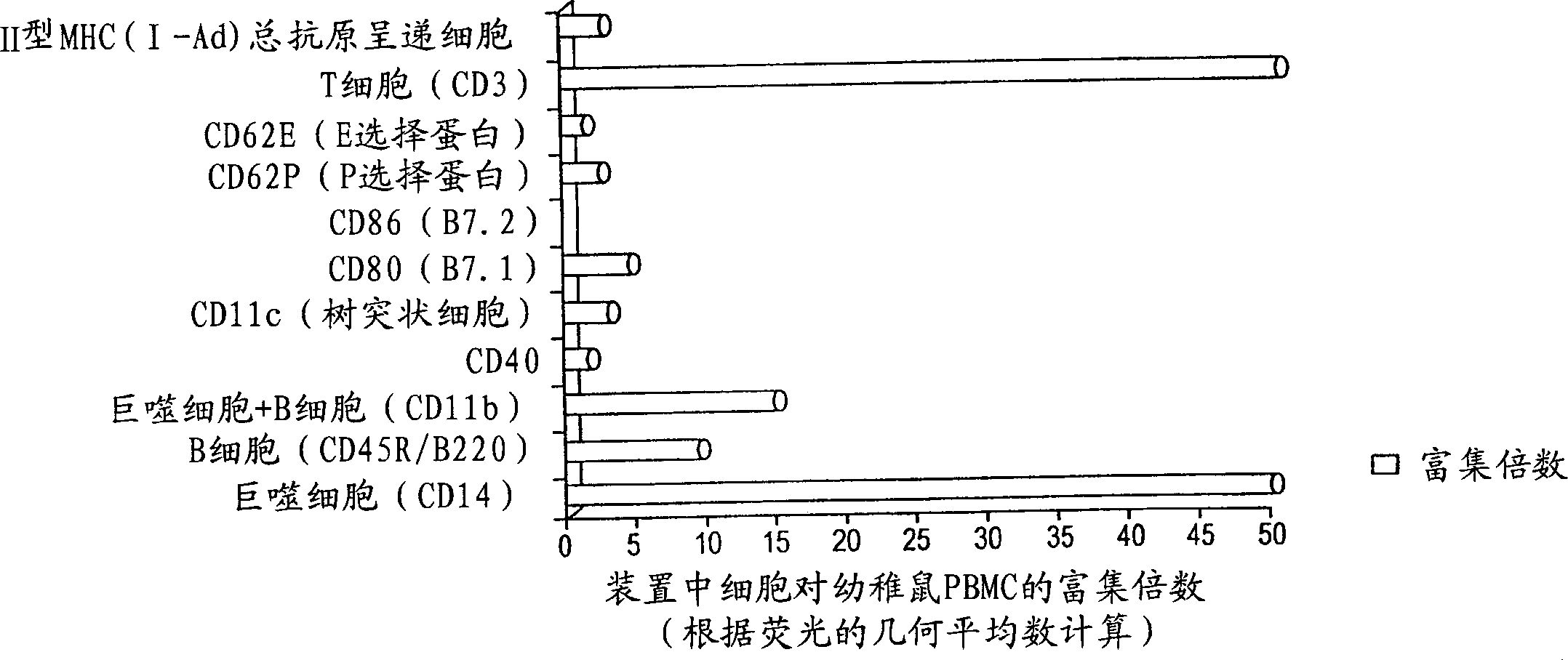
[0076] This experiment utilizes BALB / c mice to detect the number and phenotype of cells present in the device. During the 4 days following device implantation as described in Example 1, cells were aspirated from the devices of 5 animals each, pooled, washed, and distributed into several test tubes. Add fluorescein isothiocyanate or phycoerythrin-labeled monoclonal cells specific for the following markers (CD14, CD45 / B220, CD11b, CD40, CD11c, CD80, CD86, CD62P, CD62E, CD3, and I-Ad). Antibodies were cloned, incubated at 4°C for 30-45 minutes, then cells were washed and the geometric mean fluorescence was measured using a flow cytometer. For comparison, the same panel of specific antibodies was also used to measure the fluorescence of peripheral blood lymphocytes from syngeneic untreated mice and the results obtained for each antibody were expressed as the increase in fluorescence of cells from the device relative to peripheral blood lymphocytes percentage.
[0077] figure 2 ...
Embodiment 3
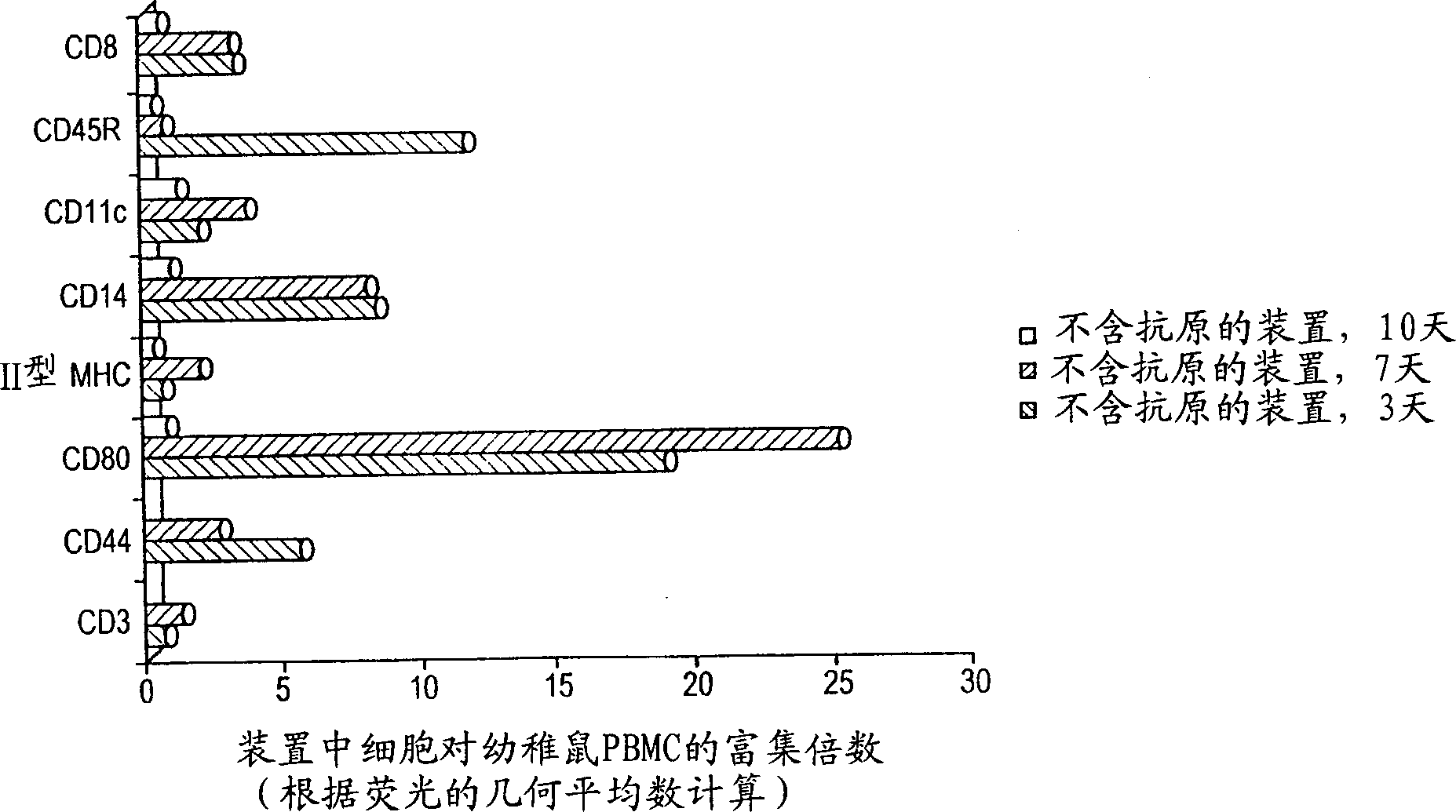
[0079] The change in cell population in the device as described in Example 1 over time was calculated by harvesting cells from the device at 3, 7 and 10 days after implantation. In the first experiment, the device did not contain any antigens. The cells aspirated from the implanted devices of 5 mice at each time point were pooled and tested for CD3, CD8, CD80, CD44, CD11c, CD45R / B220 and CD14 markers in the same manner as described in Example 2. The density of cells was expressed as the percentage increase relative to the peripheral blood lymphocytes of untreated mice.
[0080] image 3 The calculated density of cells of each phenotype at 3, 7 and 10 days post-implantation is given. For all markers, enrichment of each cell type in the device was evident on days 3 and 7, while by day 10 the cell population had migrated out of the device.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Outer diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap