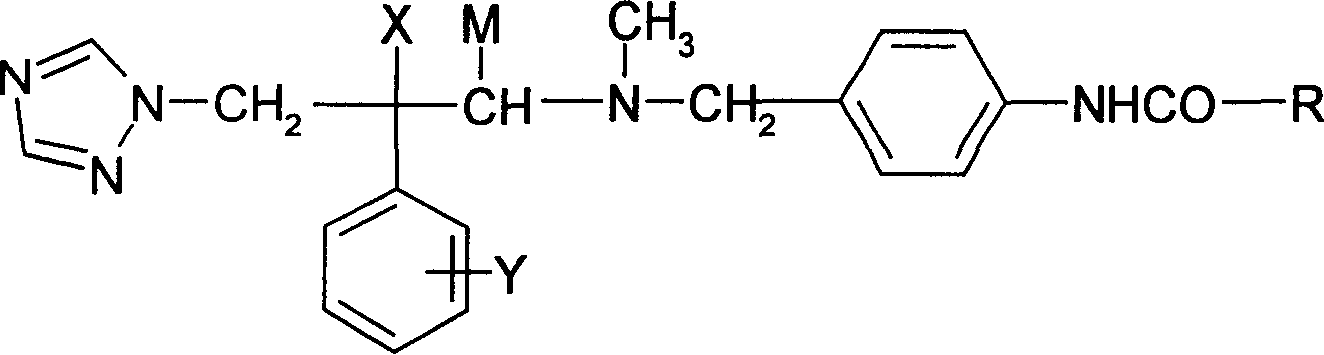
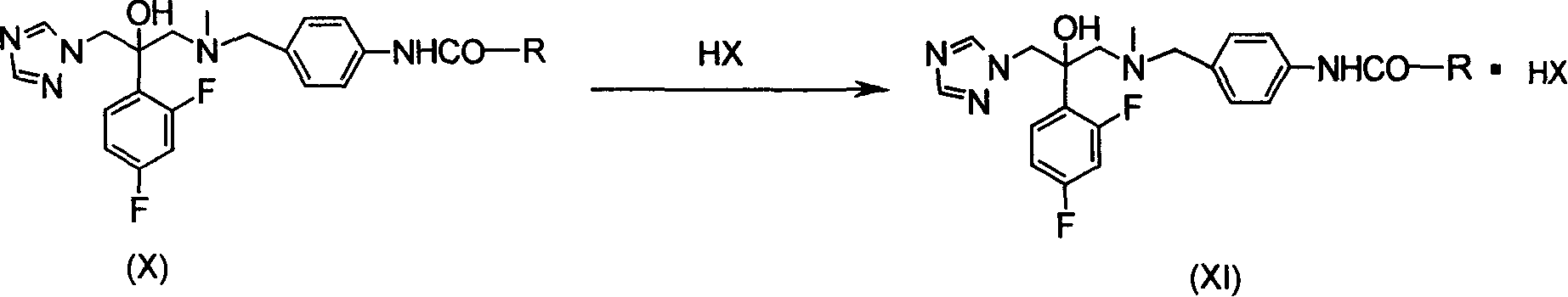
Antifungal compound of substitution benzyl triazole alcohols and preparing process thereof
A kind of technology of benzylamine triazole and compound, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
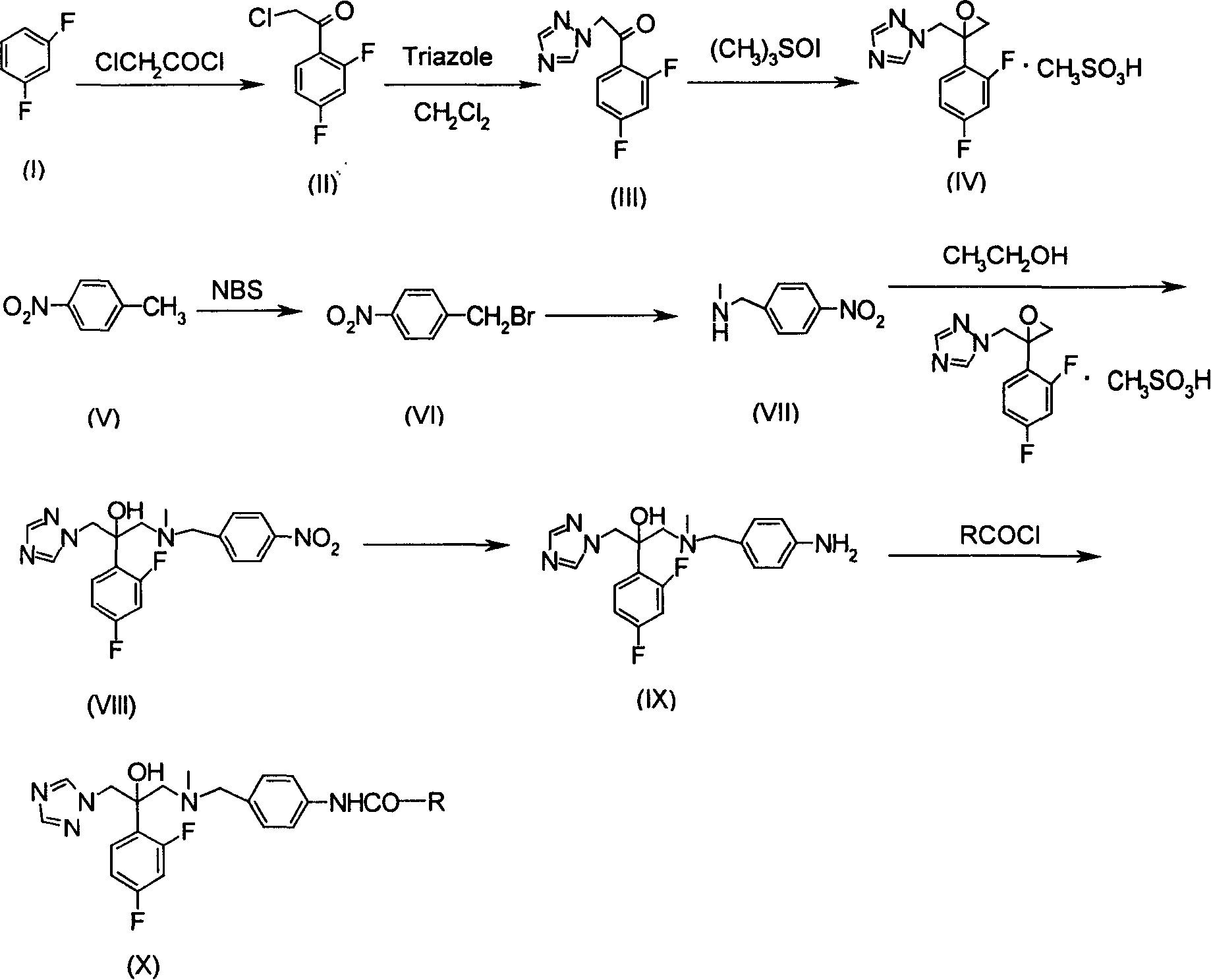
[0079] Example 1: Preparation of 2-chloro-2', 4'-difluoroacetophenone (II)
[0080] 100g (0.747mol) of anhydrous aluminum oxide and 75.33g (0.667mol) of m-difluorobenzene were placed in a 500mol three-neck flask, stirred at room temperature, and 75.33g (0.667mol) of chloroacetyl chloride was slowly added dropwise, and the dropwise addition was completed Then continue to stir at room temperature for 30 minutes, slowly raise the temperature to 50°C, continue stirring at this temperature for 5 hours, pour the reaction solution into ice water as usual, precipitate crystals, and filter to obtain a solid; the filtrate is divided into two parts with 400 mL of methylene chloride The second extraction, the combined dichloromethane extracts, washed to neutral, dried over anhydrous sodium sulfate, filtered, and the solvent was recovered to obtain a solid, the solid obtained by combining two times was recrystallized with methanol to obtain 2-chloro-2',4'- Difluoroacetophenone (II) 107.38g...
Embodiment 2
[0081] Example 2: Preparation of 2-(1H-1,2,4-triazol-1-yl)-2',4'-difluoroacetophenone (III)
[0082] Triazole 27.2g (0.4mol), TEBA0.4g, anhydrous K 2 CO 3 41.56g (0.3mol) was added to 180mL of CH 2 Cl 2 A suspension was obtained in 2-chloro-2', 4'-difluoroacetophenone (II) 38.2g (0.2mol) was dissolved in 60mL CH 2 Cl 2 Add it dropwise to the above-mentioned 180mL suspension in an ice bath, and the drop is completed in about 1.5 hours. After the drop is completed, react at 0-5°C for 5 hours, and react at room temperature for 24 hours. Then filter, filter cake with CH 2 Cl 2 Wash several times, collect the filtrate, wash the filtrate 3 times with water, each 100mL, anhydrous Na 2 SO4 is dried and CH is distilled off 2 Cl 2 , dissolve the residue in 100 mL of anhydrous ethyl acetate, stir and add concentrated nitric acid dropwise until the yellow solid no longer precipitates, filter, wash the filter cake several times with a small amount of ethyl acetate, dry it, dissol...
Embodiment 3
[0083] Example 3: Preparation of 1-[2-(2,4-difluorophenyl)-2,3-epoxypropyl]-1H-1,2,4-triazole mesylate (IV)
[0084] Get 2-(1H-1,2,4-triazol-1-yl)-2',4'-difluoroacetophenone (III) 29.8g (0.115mol), trimethyl iodine oxysulfide 25.3g ( 0.115mol), 1.6g of trimethylhexadecylammonium bromide, put into a 500mL three-necked flask, add 180mL of toluene and 225mL of 20% sodium hydroxide solution (w / w), heat at 60°C for 3 hours, and the reaction is completed Finally, the toluene layer was separated, and the water layer was extracted with toluene (100mLX2). The toluene layers were combined and washed with water until neutral. 2 mL of ethyl acetate of methanesulfonic acid precipitated a pale yellow solid, which was filtered and recrystallized from methanol as usual to obtain 21.71 g of compound (IV), with a yield of 56.7% and a melting point of 128-129°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com