High-activity blood coagulation factor VIII or VIII polypeptide variant Gly710Ala
A technology of coagulation factor and high activity, applied in the field of hemophilia, can solve problems such as high cost, achieve the effects of superior stability, improved drug efficacy, and good clinical application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
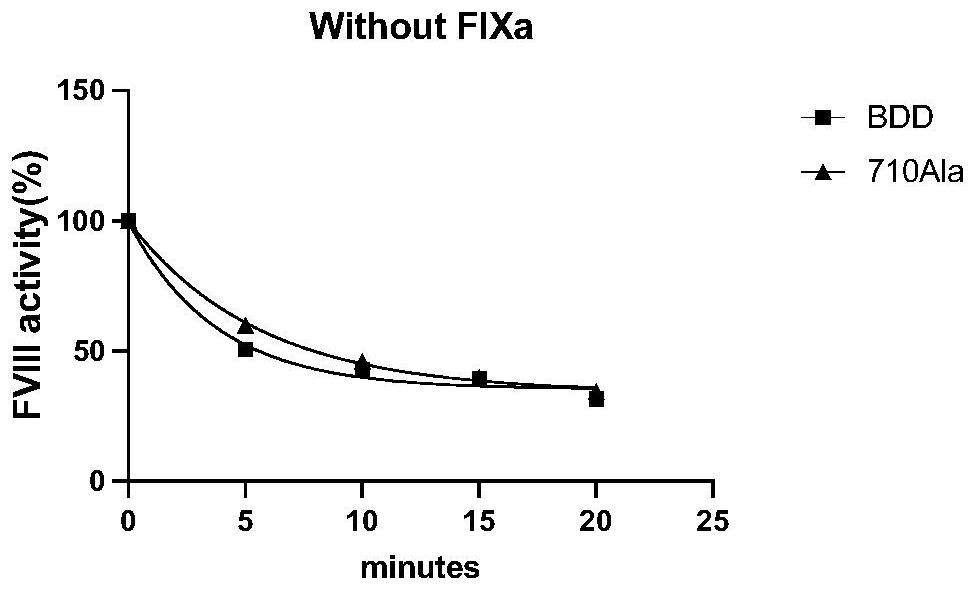
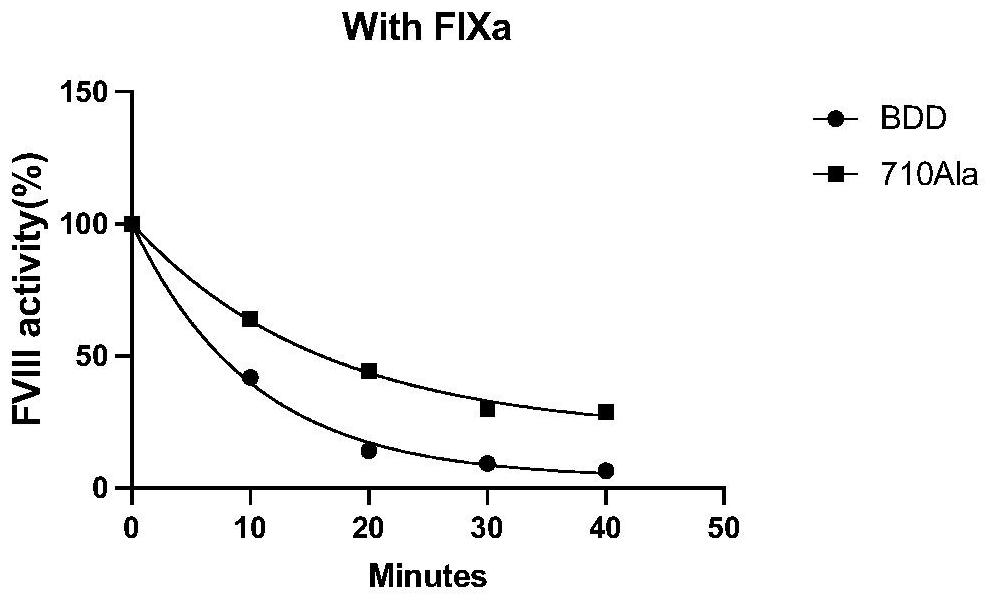
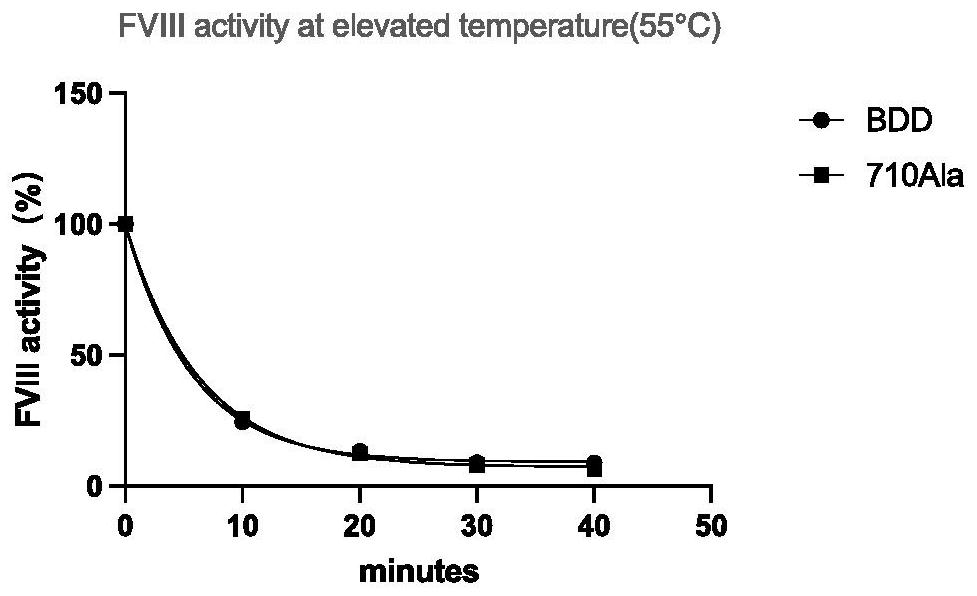
[0027] The mutant protein of highly active blood coagulation factor VIII or VIIIa polypeptide variant Gly710Ala has an amino acid sequence as shown in any one of SEQ ID NO: 21-29, and the amino acid at position 710 of the mutant is Ala instead of Gly of wild type VIII or VIIIa. In this embodiment, the mutein of hFVIII cDNA is taken as an example.
[0028] 1. Expression and purification of FⅧGly710Ala mutant
[0029] (1) Expression: using the QuikChange site-directed mutagenesis kit ( Ⅱ XL Site-Directed Mutagenesis Kit, Agilent, USA), using human B-region-deleted blood coagulation factor VIII (BDD-hFVIII) as a template, using PCR site-directed mutagenesis to introduce mutation sites into wild-type FVIII, replacing 710Gly with 710Ala , the amplified product is transformed after being digested with Dpn I (add 5-10 microliters of the mutant product digested by Dpn I to every 100 microliters of competent bacteria), and the transformed bacteria are coated to contain 1 / 2000 ampicil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com