Method for preparing thujopsanone under catalysis of MTO
A technology for the preparation of cedarnone and catalysis, which is applied in chemical instruments and methods, oxidative preparation of carbonyl compounds, catalytic reactions, etc., to achieve the effects of excellent catalytic performance, good solubility, thermal stability, and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
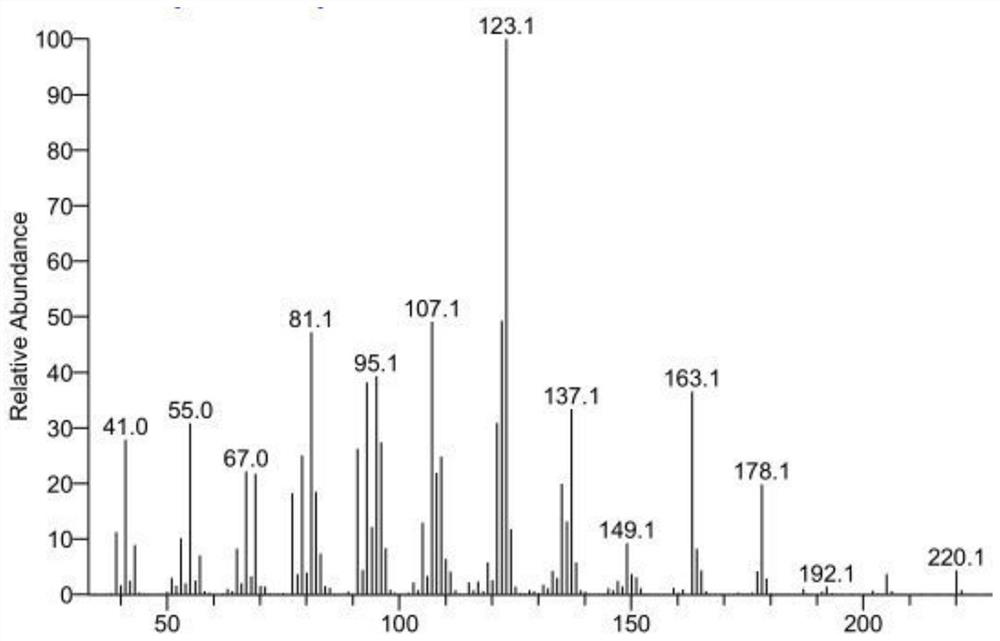
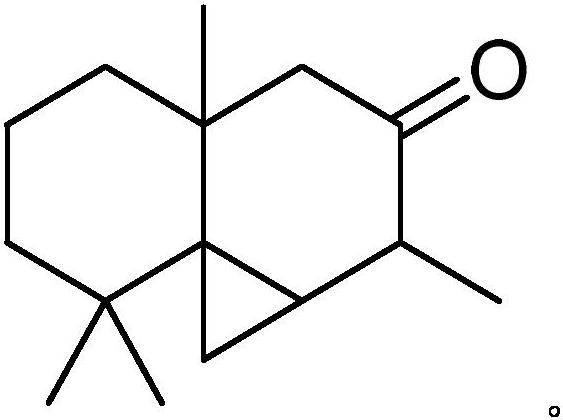
[0026] Get 6.12g pagodarene (content 68%), 0.018g methyl rhenium trioxide, 0.612g 3-methylpyrazole, 15mL ethanol, join in the reactor, and 6.8g 30%H 2 o 2 Slowly drop into the reactor, then stirred at 30°C for 3h. After the reaction was completed, the reaction solution was extracted with ethyl acetate (3*50mL); the organic phase was sequentially washed with 10% Na 2 CO 3 solution (3*50mL), deionized water (3*50mL), and saturated sodium chloride solution (3*50mL) for washing; the organic phase was dehydrated with anhydrous sodium sulfate, the solvent was recovered by distillation, and then rectified under reduced pressure to obtain Arhat The crude product 3.14g of cedrene, gas chromatographic analysis of cedrene content is 81.9%, calculated, cedrene yield is 57.3%, the conversion rate of cedrene is 76.8%; crude product by column chromatography , the purity of 94.6% was obtained, and it was determined to be luohan cedryl ketone by GC-MS characterization.
Embodiment 2
[0028] Get 6.12g pagoda ene (content 68%), 0.018g methyl rhenium trioxide, 0.612g 3-methylpyrazole, 15mL methylene chloride, join in the reactor, and 6.8g 30%H 2 o 2 Slowly drop into the reactor, then stirred at 30°C for 3h. After the reaction was completed, the reaction solution was extracted with ethyl acetate (3*50mL); the organic phase was sequentially washed with 10% Na 2 CO 3 solution (3*50mL), deionized water (3*50mL), and saturated sodium chloride solution (3*50mL) for washing; the organic phase was dehydrated with anhydrous sodium sulfate, the solvent was recovered by distillation, and then rectified under reduced pressure to obtain Arhat The crude product 3.53g of cedrene, gas chromatographic analysis of cedrene content is 76.8%, calculated, cedrene yield is 60.4%, the conversion rate of cedrene is 80.7%; crude product is passed through column chromatography , the purity of 93.7% was obtained, and it was determined to be luohancypressanone through GC-MS characteri...
Embodiment 3
[0030] Get 6.12g pagodarene (content 68%), 0.018g methyl rhenium trioxide, 0.612g 3-methylpyrazole, 15mL ethyl acetate, join in the reactor, and 6.8g 30%H 2 o 2 Slowly drop into the reactor, then stirred at 30°C for 3h. After the reaction was completed, the reaction solution was extracted with ethyl acetate (3*50mL); the organic phase was sequentially washed with 10% Na 2 CO 3 solution (3*50mL), deionized water (3*50mL), and saturated sodium chloride solution (3*50mL) for washing; the organic phase was dehydrated with anhydrous sodium sulfate, the solvent was recovered by distillation, and then rectified under reduced pressure to obtain Arhat The crude product 3.84g of cedrene, gas chromatographic analysis of cedrene content is 73.2%, calculated, cedrene yield is 62.6%, the conversion rate of cedrene is 81.3%; crude product is passed through column chromatography , the purity of 94.1% was obtained, and it was determined to be luohan cedrynone through GC-MS characterization....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com