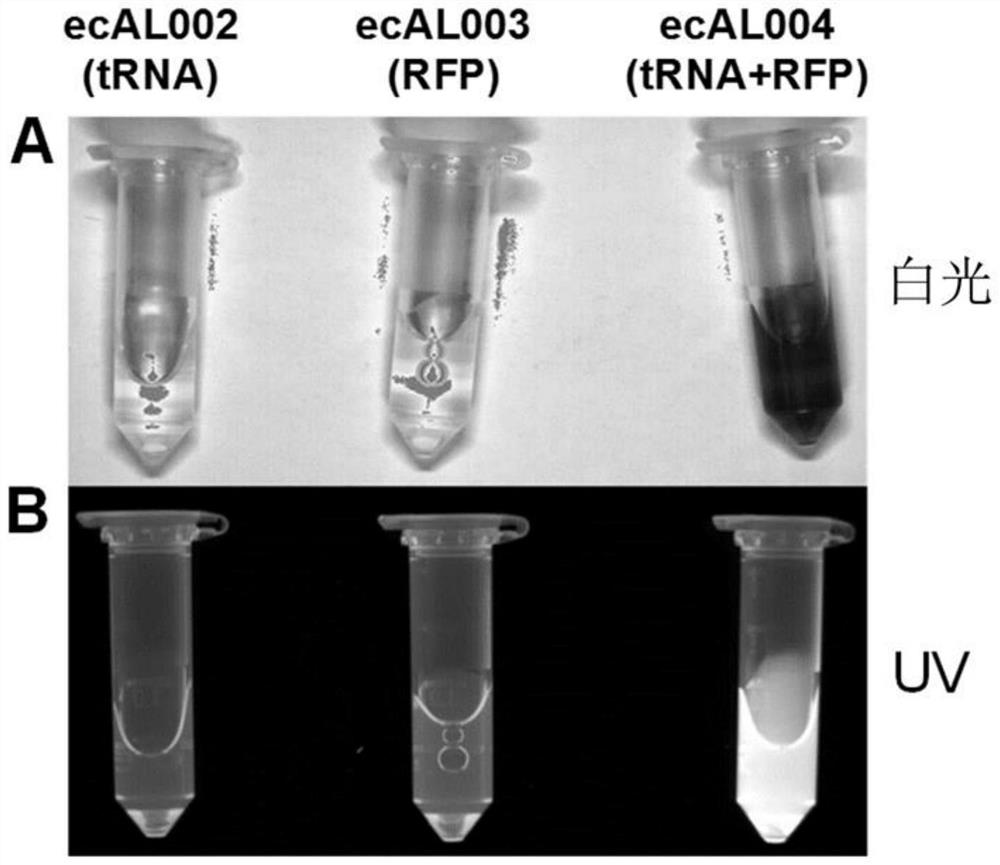
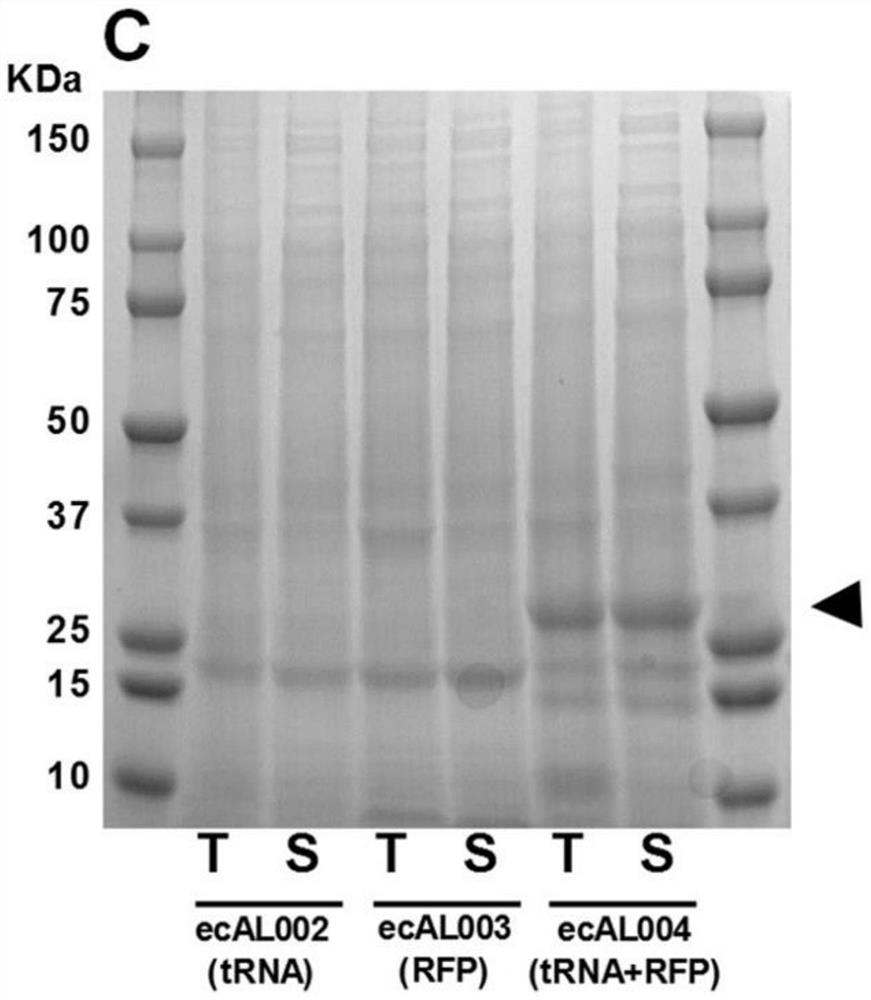
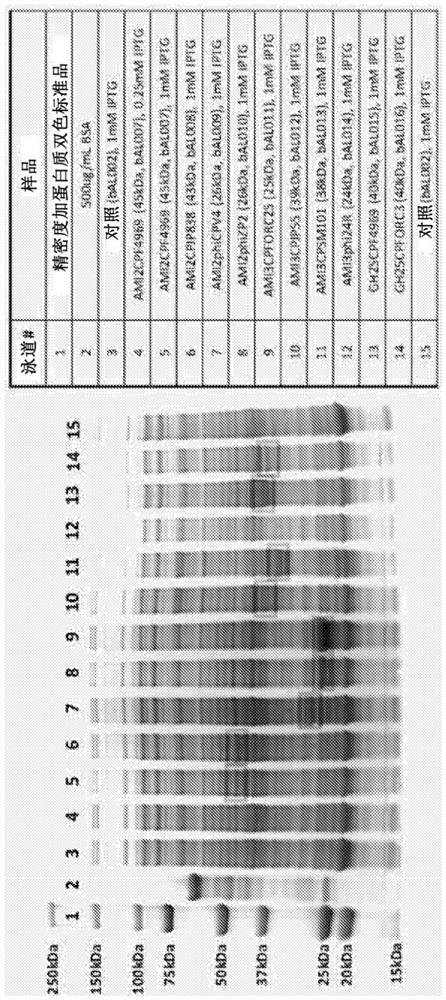
Antimicrobial endolysin polypeptides, compositions and formulations
An anti-microbial, endolysin technology, applied in the field of host cells, can solve the problems of affecting pathogenic bacteria, adverse effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0286] 1. Inoculate Clostridium perfringens strain NCTC 8237 into BHI+C medium, and culture at 37°C under anaerobic conditions overnight to the stationary phase;
[0287] 2. Inoculate the overnight culture into fresh BHI+C medium, and culture it at 37°C under anaerobic conditions until the OD600nm is about 0.6;
[0288] 3. Centrifuge 1.8 mL of the culture at 16600×g for 5 minutes at room temperature under anaerobic conditions;
[0289] 4. Remove the supernatant, and resuspend the cell pellet in 0.9 mL resuspension buffer (NaCl127mM, NaCl1 2 HPO 4 70mM, NaH 2 PO 4 30mM pH 7.0);
[0290] 5. Dilute the cell suspension to OD 600nm 0.6 with resuspension buffer under anaerobic conditions;
[0291] 6. Add the purified endolysin polypeptide or fragment to a concentration of 5 μg / mL under anaerobic conditions;
[0292] 7. Incubate at 25°C for 1 hour under anaerobic conditions;
[0293] 8. Determine viable cell counts by serial dilution, plating cells on RCM solid medium, and i...
Embodiment 12
[0307] 1. Inoculate Clostridium perfringens strain NCTC 8237 into BHI+C medium, and culture at 37°C under anaerobic conditions overnight to the stationary phase;
[0308] 2. Inoculate the overnight culture into fresh BHI+C medium and culture under anaerobic conditions at 37°C until the culture reaches exponential phase (OD 620nm about 1.0);
[0309] 3. Centrifuge a certain volume of exponential phase culture at 3800×g for 30 minutes at 10°C, then remove the supernatant;
[0310] 4. Resuspend the cells in a volume of PBS (pH 7.0) that is half of the initial pre-centrifuged culture volume to concentrate the cells;
[0311] 5. Transfer 90 μL of resuspended cells to a well of a 96-microwell plate, and add 10 μL of a 10-fold concentrated endolysin polypeptide or fragment in PBS (pH 7.0) to a final concentration of 5 μg / mL;
[0312] 6. Monitor the decrease in turbidity by taking OD 620nm kinetic readings at appropriate intervals on a microplate reader at room temperature;
[0313]...
Embodiment 15
[0320] 1. Inoculate Clostridium perfringens strain Cp6 into BHI+C medium, and culture at 37°C under anaerobic conditions overnight to the stationary phase;
[0321] 2. Dilute the stationary phase culture in fresh LB medium under anaerobic conditions;
[0322] 3. Under anaerobic conditions, prepare serial dilutions of the purified endolysin stock solution in PBS (pH 7.0) to a concentration 10 times higher than the final desired protein loading concentration;
[0323] 4. Under anaerobic conditions, mix serial dilutions of purified endolysin with diluted stationary phase cultures in wells of a microtiter plate, where, for each well, mix 45 μL of diluted stationary phase cultures with 5 μL of 10-fold concentrated and purified endolysin was mixed to a final cell load of approximately 10 4 cells / mL;
[0324] 5. Incubate the cells overnight at 41°C for 12-20 hours under anaerobic conditions;
[0325] 6. Determine the degree of growth inhibition by measuring the change in OD on a m...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap