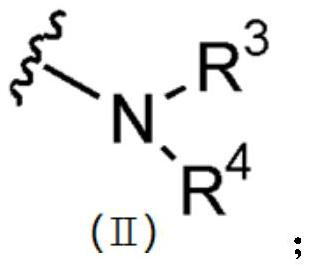
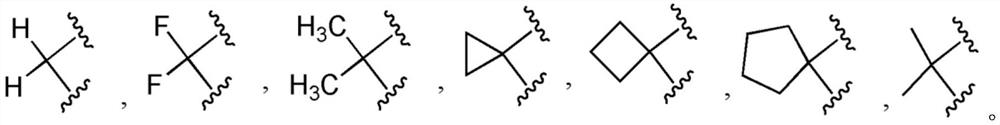
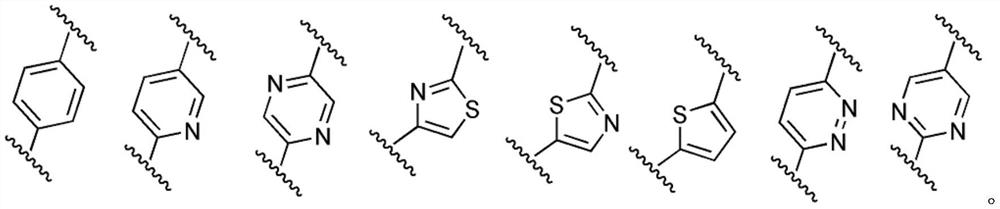
5-aminoisoxazole derivative and application thereof in preparation of multi-kinase inhibitor
A technology of aminoisoxazole and derivatives, applied in the field of 5-aminoisoxazole derivatives and its application in the preparation of multi-kinase inhibitors, which can solve the problems of reduced absorption, unstable action, low water solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Example 1: 2-(4-(5-(1H-pyrazol-4-yl)-1H-benzo[d]imidazol-1-yl)phenyl)-N-(isoxazol-5-yl) ) Synthesis of Acetamide (I-1)
[0106] Using intermediate 8a and N-Boc pyrazole borate derivative (10c) to react to obtain the target product. Product Characterization Results:
[0107] MS: m / z: 385.1 (M+H)+.
Embodiment 2
[0108] Example 2: 2-(4-(5-(1H-pyrazol-4-yl)-1H-benzo[d]imidazol-1-yl)phenyl)-N-(3-methylisoxazole Synthesis of -5-yl)acetamide (I-2)
[0109] The desired product was obtained using intermediate 8b and the N-Boc pyrazole borate derivative (10c).
[0110] MS: m / z: 399.2 (M+H)+.
Embodiment 3
[0111] Example 3: N-(3-(tert-butyl)isoxazol-5-yl)-2-(4-(5-(1-methyl-1H-pyrazol-4-yl)-1H-benzene Synthesis of [d]imidazol-1-yl)phenyl)acetamide (I-3)
[0112] The desired product was prepared using intermediate 8c and N-methylpyrazoleboronic acid (9b).
[0113] MS:m / z:455.2(M+H) + ; 1 H NMR(DMS:O-d6,400MHz):δ=11.87(s,1H),8.53(s,1H),8.17(s,1H),7.97(s,1H),7.92(s,1H),7.68 -7.65(m,2H),7.59-7.56(m,4H),6.23(s,1H),3.88(s,3H),3.83(s,2H),1.25(s,9H); 13 C NMR (DMS: O, 100MHz): δ=172.9, 167.9, 161.4, 145.0, 144.0, 136.5, 135.2, 134.9, 132.1, 131.3, 128.1, 127.9, 123.8, 122.8, 121.8, 116.3, 111.4, 81.4, 42. 32.4, 29.4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com