An Active Peptide with Bitter Taste Blocking Effect
A technology of blocking action and active peptides, which is applied in the directions of medical preparations, peptides, and applications of non-active ingredients, can solve limited problems, and achieve the effect of wide application prospects and good water solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] This example screens and identifies a tetrapeptide DDNK that has a significant inhibitory effect on the combination of bitter substances and T2R14 from egg white, including the following steps:
[0028] (1) Virtual enzymolysis and preliminary screening of egg white protein.
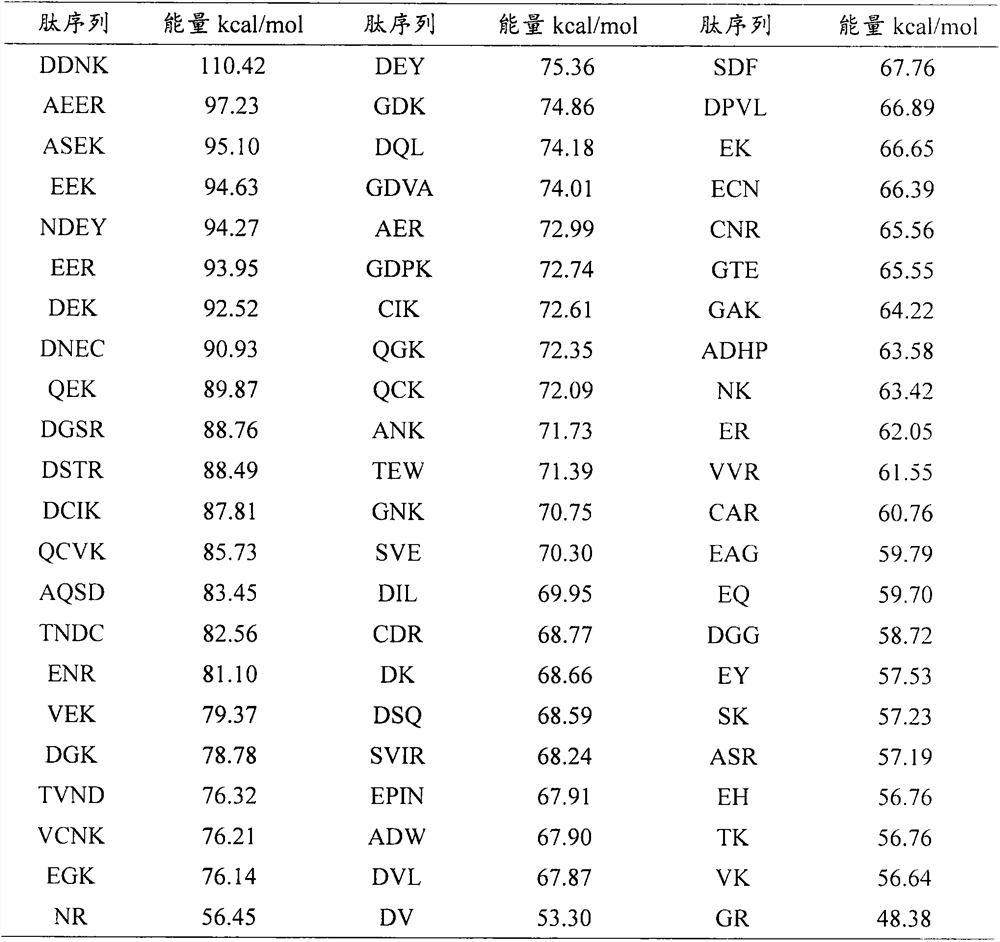
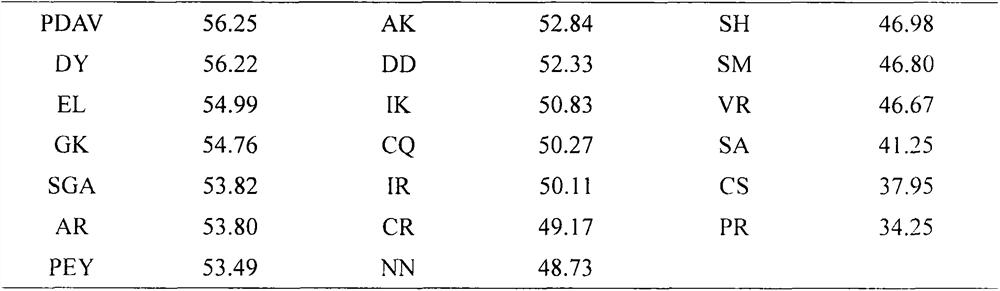
[0029] Based on the online program ExPASy PeptideCutter, ovalbumin in egg whites was treated with typical digestive proteases of the gastrointestinal tract, namely pepsin (EC 3.4.23.1), trypsin (EC 3.4.21.4) and chymotrypsin (EC 3.4.21.1). , ovotransferrin and ovomucoid were subjected to simulated enzymatic hydrolysis, and their amino acid sequences were obtained through the NCBI website (https: / / www.ncbi.nlm.nih.gov / ), and their numbers were AAB59956.1, CAA26040.1 and ACJ04729.1. 219 dipeptides and above active peptides were obtained. Unreported dipeptides, tripeptides and tetrapeptides were selected to predict toxicity and water solubility properties using the ADMET program of DS 2017 software ...
Embodiment 2
[0035] Example 2. Determination of the Blocking Activity of Active Peptides to Bitter Substances
[0036]The bitter taste inhibitory activity of a certain concentration of peptide DDNK was determined by electronic tongue. Take a certain amount of peptide DDNK, add it to 30mL water and mix well, add 50mL quinine (1mM) to prepare the solution to be tested, use quinine as the blank control group, and use the peptides LELNQ and LEGSLE as the positive control group. After setting the number of cycles, data file name, sample quantity, sample name and test method, install the electrode and start the measurement.
[0037] The measurement process is as follows: (1) Clean the sensor in the positive and negative solutions for 90s, and then clean the sensor in the two reference solutions for 120s; (2) Balance the sensor in the conditioning solution for 30s; (3) Measure each sample for 30s; (4 ) After cleaning the sensor twice for 3s each, immerse it in the reference solution for 30s to m...
Embodiment 3
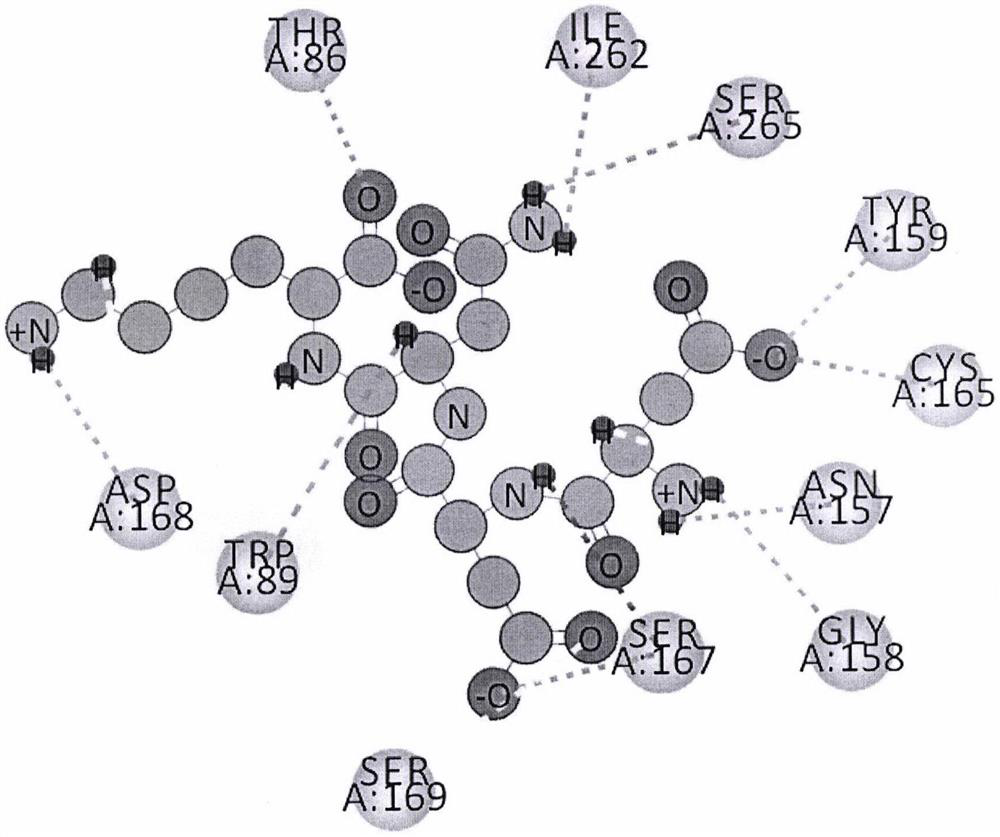
[0039] Example 3. DS software analyzes the interaction mode between peptide DDNK and T2R14.
[0040] The two-dimensional plan between peptide DDNK and T2R14 as figure 1 shown by figure 1 It can be seen that the tetrapeptide DDNK can interact with Asp168 residue, Trp89 residue, Ser167 residue, Gly158 residue, Asn157 residue, Cys165 residue, Tyr159 residue, Ser265 residue, Ile262 residue and Thr86 residue of T2R14 effect;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com