tk1 antibody, kit and use thereof
A kit and antibody technology, applied in the field of immunology, can solve the problems of prolongation of life and high resistance of liver cancer to chemotherapy, and achieve the effect of increasing the frequency of apoptosis, reducing the rate and high affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
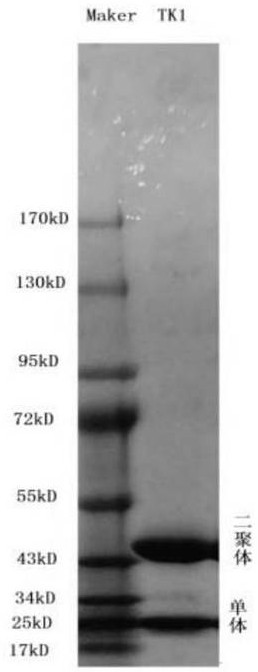
[0038] Step 1: Preparation of TK1 antigen
[0039] Inoculation: Synthesize the full-length gene sequence of TK1 into the vector pET21a-TK1-C6His, and inoculate the pET21a-TK1 BL21 expressing bacteria into 5 ml of ampicillin-resistant LB medium from a -80 °C refrigerator, and cultivate overnight at 37 °C and 220 rpm (do not thaw the expression bacteria during inoculation). ).
[0040] Mass induction: the next day, inoculate BL21 (DE3) bacterial solution in 1000 ml of ampicillin-resistant TB medium at a ratio of 1:200. Cultivate at 37°C and 220rpm. When the bacteria enter the logarithmic growth phase (OD is about 0.6, the cultured bacteria are just invisible, in a cloudy state), the culture flask is cooled in an ice-water mixture, and IPTG with a final concentration of 1mM is added. Induction for 15 hours.
[0041] To collect expressing bacteria: the bacterial solution was centrifuged at 6000 rpm for 15 min to remove the supernatant, the bacteria were resuspended in 0.9% salin...
Embodiment 1
[0114] Step 1: Fully mix the liver tumor cell lines Hep G2 and Hep 3B with the 0.1 μg / ml monoclonal antibody containing the SSTK mouse hybridoma cell line 43# of the present invention (2×10 4 ), filter sterilized in a biosafety cabinet with a 0.22um sterile filter (Cat. No. SLGP033RB), cultured in MEM Alpha basic cells containing 15% fetal bovine serum (ExCell Bio, Cat. No. FS500) without antibiotics or other reagents Culture medium (GIBCO, catalog C12571500BT) for 72h;
[0115] Step 2: The liver tumor cell lines Hep G2, Hep 3B 0.22um were filter sterilized and then cultured in MEM Alpha basic cells containing 15% fetal bovine serum (ExCell Bio, catalog number FS500) without antibiotics or other reagents culture medium (GIBCO, catalog C12571500BT) for 72h;
[0116] Step 3: Carry out cell viability test (Cell Counting Kit-8, MedChemExpress)
[0117] Viability calculation: tumor cell viability (%) = (absorbance of experimental well - absorbance of blank medium control) / (abso...
Embodiment 2
[0119] Step 1: Fully mix the liver tumor cell lines Hep G2 and Hep 3B with 1 μg / ml monoclonal antibody containing the SSTK mouse hybridoma cell line 43# of the present invention (2 × 10 4 ), cultured in MEM Alpha basic cell culture medium (GIBCO, catalog C12571500BT) containing 15% fetal bovine serum (ExCell Bio, catalog number FS500) without antibiotics or other reagents for 72 h;
[0120] Step 2: Hepatic tumor cell lines Hep G2, Hep 3B were cultured in MEM Alpha basic cell culture medium (GIBCO, cat. C12571500BT) containing 15% fetal bovine serum (ExCell Bio, cat. no. FS500) without antibiotics or other reagents 72h;
[0121] Step 3: Carry out cell viability test (Cell Counting Kit-8, MedChemExpress) Viability calculation: tumor cell viability (%)=(absorbance of experimental well-absorbance of blank medium control) / (absorbance of untreated control well- Blank medium control absorbance) × 100%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com