Implantable urine control structure
An implantable, urethral technology, applied in the field of medical devices, can solve the problems of large surgical trauma, large urethral binding force, and difficult to idealize the tightness adjustment, so as to improve the safety of use, improve the comfort of use, and relieve poor blood flow Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0037]In order to enable those skilled in the art to better understand the technical solutions in the embodiments of the present application, the following will clearly and completely describe the technical solutions in the embodiments of the present application in conjunction with the drawings in the embodiments of the present application. Obviously, the described The embodiments are only some of the embodiments of the present application, but not all of them. All other embodiments obtained by persons of ordinary skill in the art based on the embodiments in the embodiments of the present application shall fall within the protection scope of the embodiments of the present application.
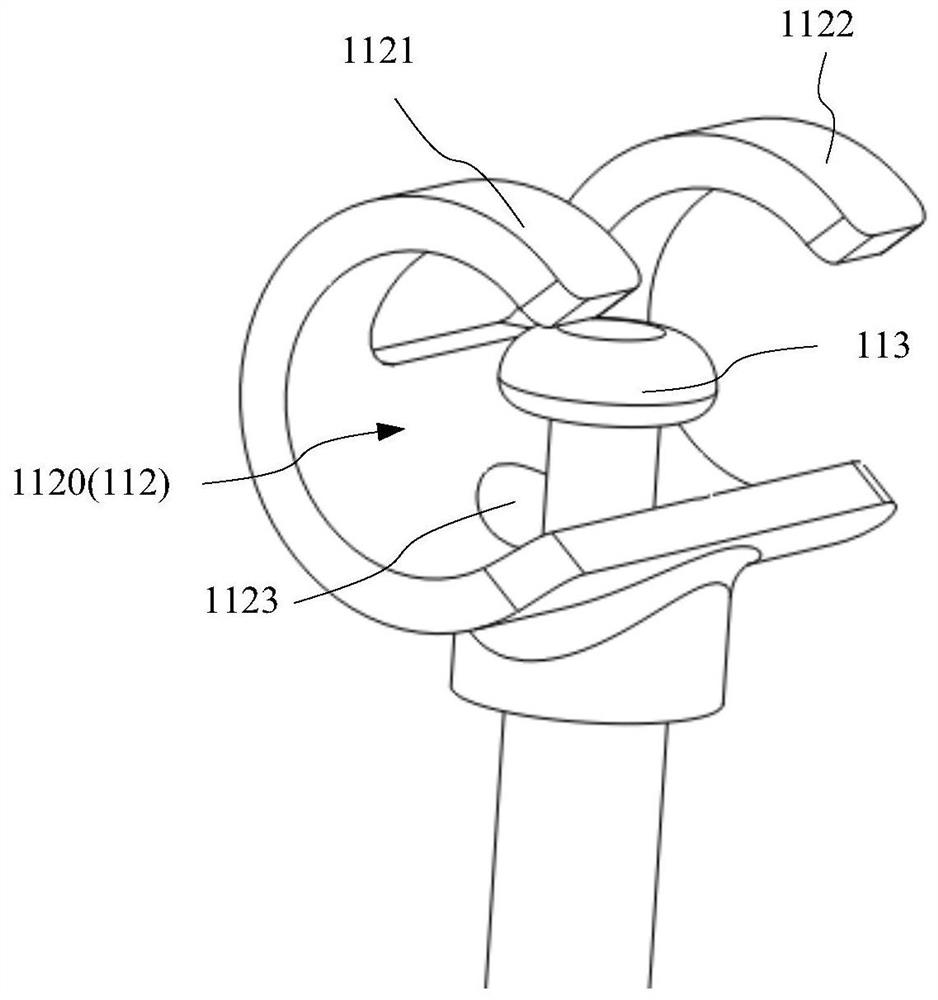
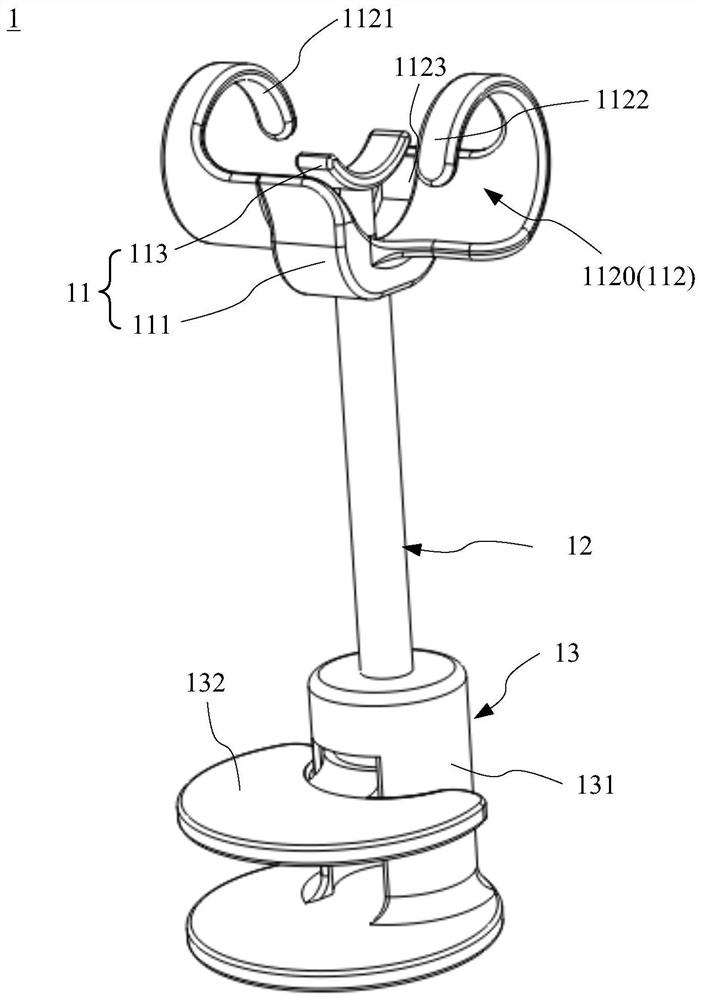
[0038] Figure 1A and Figure 1B It is a schematic diagram of the overall structure of the implantable urinary control structure 1 of the first embodiment of the present application, wherein, Figure 1A It is a schematic diagram showing that the urinary control assembly 11 is in a bound state, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com