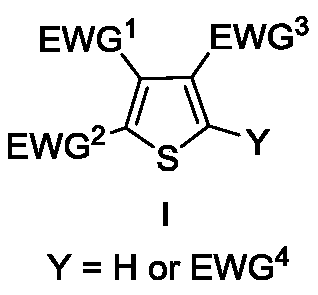
Method for preparing polysubstituted thiophene
A multi-substitution and thiophene technology, applied in organic chemistry and other fields, can solve problems such as rare literature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The reaction formula of embodiment 1, the specific raw material compound A-1 and B-1 used and the structure of product I-1 are as follows
[0020]
[0021] The specific experimental steps are: 127mg (0.45mmol, 1.5eq) of compound A-1 and 56mg (0.3mmol, 1.0eq) of compound B-1 were dissolved in 3mL of dichloroethane and reacted at 85°C. Reaction monitoring B-1 disappeared completely, and the reaction was completed, and the reaction mixture was rotary-evaporated under the reduced pressure of the water pump to remove the solvent dichloroethane. The residue was chromatographed on a 200-300 mesh silica gel column to obtain the compound shown in I-1, and the product was identified by NMR (hydrogen spectrum, carbon spectrum) and high-resolution mass spectrometry.
[0022] Product Ⅰ-1 was a yellow oil with a yield of 81%. 1 H NMR (400MHz, CDCl 3 )δ7.79(d, J=7.3Hz, 2H), 7.60(t, J=7.4Hz, 1H), 7.46(t, J=7.7Hz, 2H), 3.95(s, 3H), 3.69(s, 3H), 3.68(s, 3H); 13 CNMR (100MHz, CDCl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com