A kind of thioether compound and its preparation method, pharmaceutical intermediate and its application
A compound and thioether technology, which is applied in the field of thioether compounds and their preparation, can solve problems such as metal residues, achieve the effects of eliminating metal residues, mild reaction conditions, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
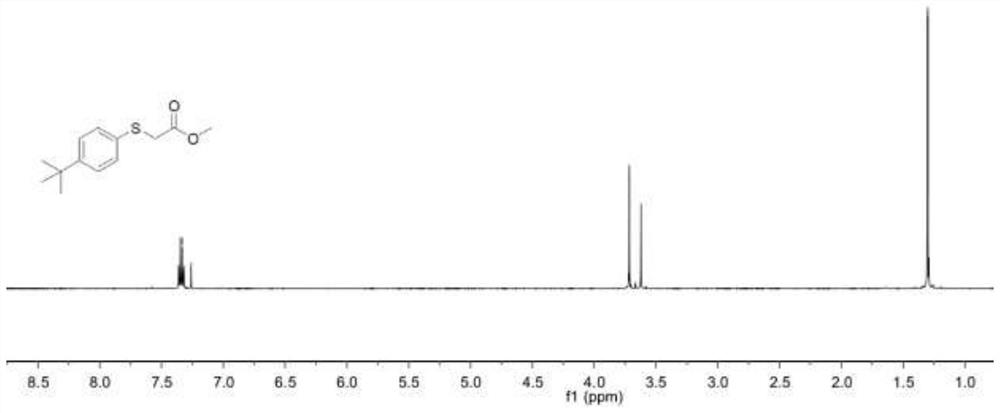
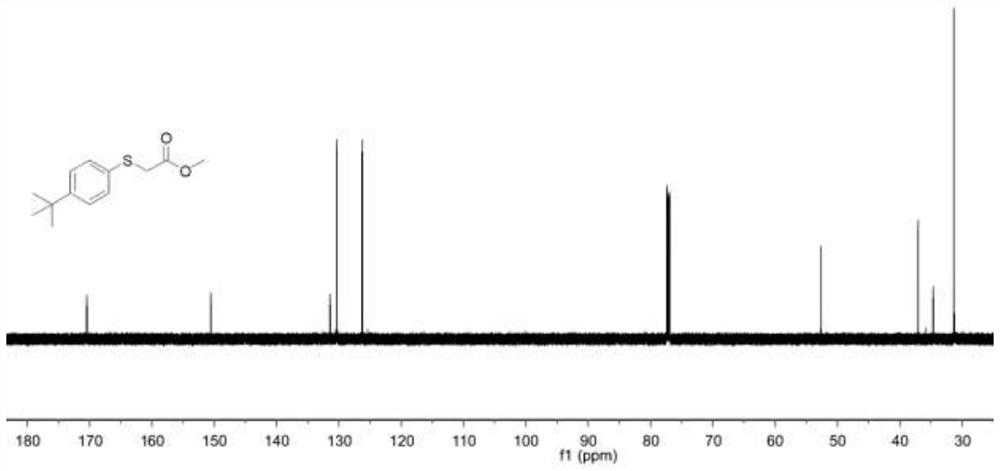
Embodiment 1
[0045] A thioether compound 001, namely the thioether compound numbered 001, the specific structural formula is:
[0046]
[0047] In the present embodiment, the specific synthesis steps of the thioether compound 001 are as follows:
[0048] 1-(tert-Butyl)-4-(methylsulfoxide)benzene (60.1 mg, 0.3 mmol), followed by methyl bromoacetate (281.0 mg) were added to a 4 mL pre-dried vial at room temperature. , 1.8 mmol), at the same time, 0.15 mL of 1-butyl-3-methylimidazole trifluoromethanesulfonate was added, and then placed in a reactor at 100 ° C for 48 h; the reaction system after the reaction was cooled to room temperature ( Usually 25°C), after cooling, the reaction system was washed with water, then extracted three times with ethyl acetate, and the organic phases extracted three times were combined into a 50 mL eggplant-shaped bottle, and a Heidolph rotary evaporator (rotating speed was 90 rpm, temperature was 40 ℃, vacuum degree is 0.1Mpa) rotary evaporation for 3min, th...
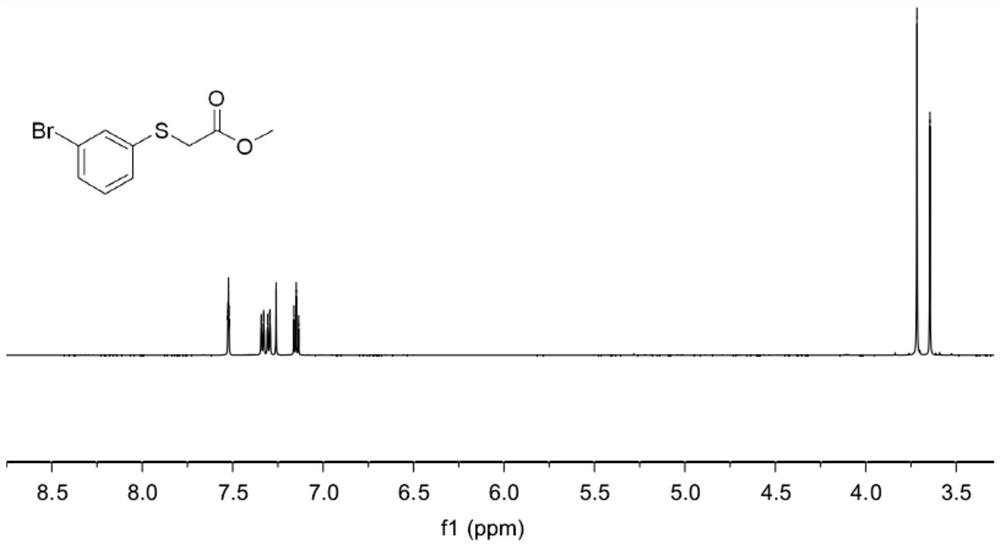
Embodiment 2
[0054] A thioether compound 002, namely the thioether compound numbered 002, the specific structural formula is:
[0055]
[0056] In this embodiment, the specific synthesis steps of the thioether compound 002 are as follows:
[0057] 1-Bromo-3-(methylsulfoxide)benzene (67.1 mg, 0.3 mmol), followed by methyl bromoacetate (281.0 mg, 1.8 mmol) were added to a 4 mL pre-dried vial at room temperature. , at the same time, 0.15 mL of 1-butyl-3-methylimidazole trifluoromethanesulfonate was added, and then placed in a reactor at 100 ° C for 48 h; the reaction system after the reaction was cooled to room temperature (usually 25 ° C ), the reaction system was washed with water after cooling, and then extracted three times with ethyl acetate, and the organic phase extracted three times was merged into a 50 mL eggplant-shaped flask, and a Heidolph rotary evaporator (rotating speed was 90 rpm, temperature was 40 ° C, vacuum 0.1Mpa) rotary evaporation for 3min, the residue was separated...
Embodiment 3
[0063] A thioether compound 003, namely the thioether compound numbered 003, the specific structural formula is:
[0064]
[0065] In the present embodiment, the specific synthesis steps of the thioether compound 003 are as follows:
[0066] 4-(Methylsulfoxide)benzaldehyde (51.5 mg, 0.3 mmol), methyl bromoacetate (281.0 mg, 1.8 mmol) were sequentially added to a pre-dried vial with a capacity of 4 mL at room temperature, and simultaneously 0.15mL of 1-butyl-3-methylimidazole trifluoromethanesulfonate, and then placed in a reactor at 100°C for 48h; the reaction system after the reaction was cooled to room temperature (usually 25°C), cooled After the completion, the reaction system was washed with water, then extracted three times with ethyl acetate, and the organic phases extracted three times were combined into a 50 mL eggplant-shaped bottle, and a Heidolph rotary evaporator (rotation speed was 90 rpm, temperature was 40 ° C, and vacuum degree was 0.1 Mpa) rotary evaporati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com