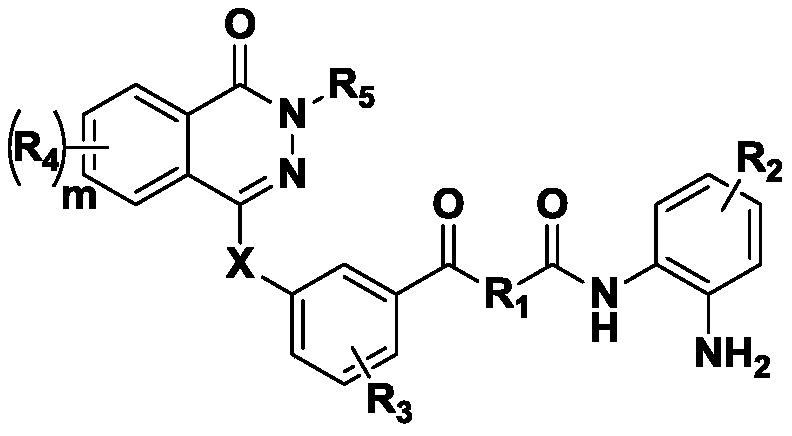
Phthalazine derivative, preparation method and applications thereof
A derivative, phthalazine technology, applied in the field of phthalazine derivatives and its preparation, can solve the problems of complex pharmacokinetics, adverse reactions, drug interactions, inability to completely kill tumor cells, and easy drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
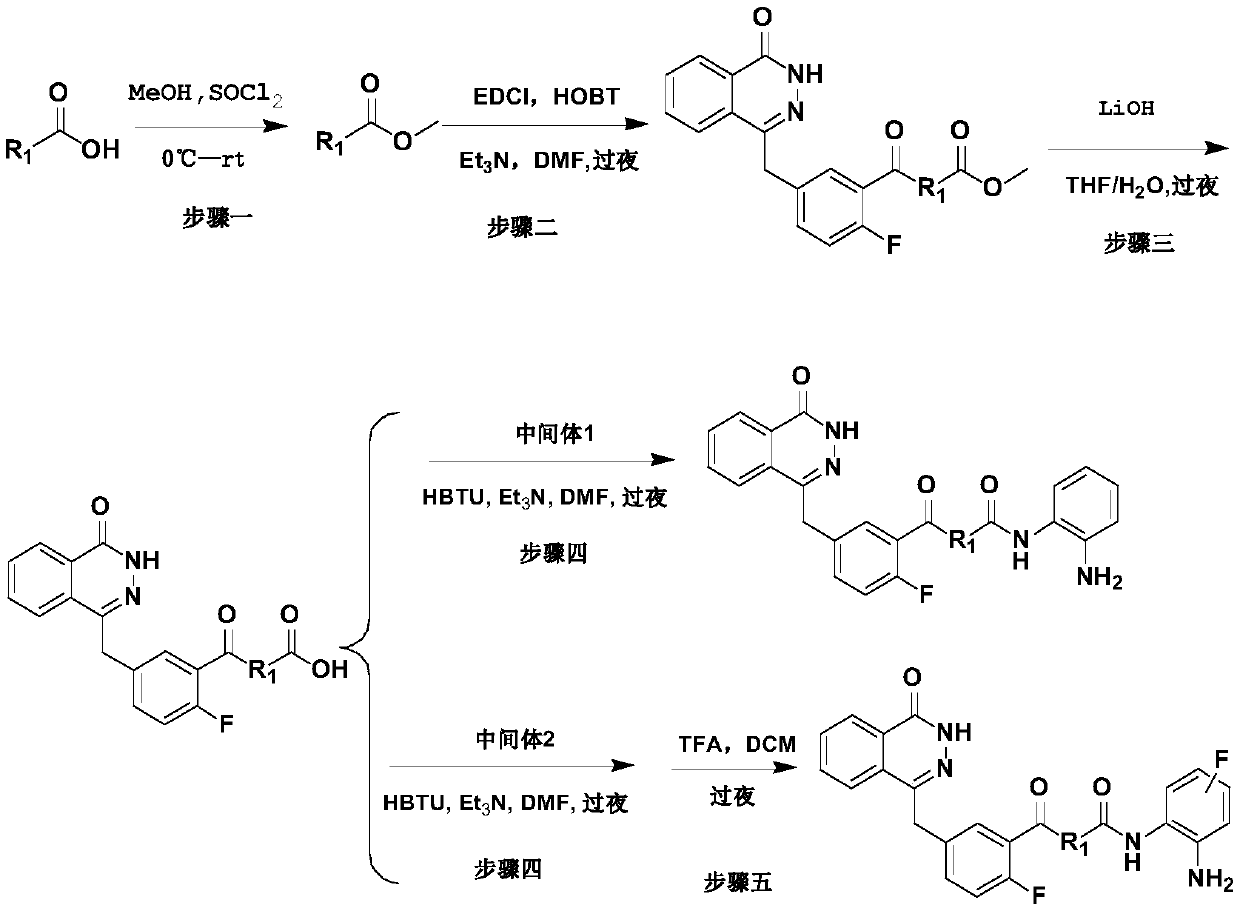
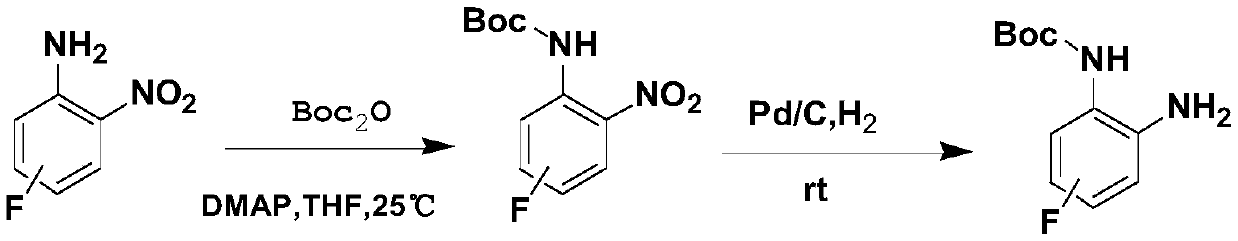
[0042] N-(4-((2-aminophenyl)carbamoyl)benzyl)-2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl) Preparation of benzamide (compound 1):
[0043]
[0044] Step 1: Synthesis of 4-(aminomethyl) methyl benzoate
[0045] Dissolve 1 g of 4-(aminomethyl)benzoic acid (0.0066 mol) in 20 ml of methanol, and stir for 5 mins under ice-cooling. Slowly add 0.71ml of thionyl chloride dropwise, after the drop is complete, stir in an ice bath for 10mins, then raise the temperature to 60°C, and stir at reflux overnight. After the completion of the reaction as monitored by TLC, the methanol was evaporated under reduced pressure to obtain 0.98 g of a white solid after drying, with a yield of 90.5%, and the product was directly used in Step 2.
[0046]Step 2: Synthesis of methyl 4-((2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)benzamido)methyl)benzoate
[0047] 0.5g 2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)benzoic acid (0.0017mol), 0.35g1-(3-dimethylaminopropyl )-3-Et...
Embodiment 2
[0053] N-((4-((2-aminophenyl)carbamoyl)cyclohexyl)methyl)-2-fluoro-5-((4-oxo-3,4-dihydro-phthalazine-1- base) methyl) benzamide (compound 2)
[0054]
[0055] According to the preparation method of compound 1, tranexamic acid was used instead of 4-(aminomethyl)benzoic acid, and the rest of the operations were the same. The product is a white solid. m.p.243℃-246℃. 1 H NMR (600MHz, DMSO-d 6 )δ (ppm): 12.56 (s, 1H), 8.98 (s, 1H), 8.27–8.17 (m, 2H), 7.96 (d, J = 8.0Hz, 1H), 7.87 (t, J = 7.6Hz, 1H), 7.81(t, J=7.5Hz, 1H), 7.51(d, J=5.3Hz, 1H), 7.42(s, 1H), 7.20–7.11(m, 2H), 6.86(t, J=7.6 Hz, 1H), 6.69(d, J=7.8Hz, 1H), 6.51(t, J=7.5Hz, 1H), 4.75(s, 2H), 4.31(s, 2H), 3.09(t, J=6.1 Hz, 2H), 2.30(t, J=12.0Hz, 1H), 1.86(d, J=12.1Hz, 2H), 1.79(d, J=11.8Hz, 2H), 1.49(s, 1H), 1.44– 1.33(m,2H),0.97(q,J=11.7Hz,2H).HRMS(ESI-TOF):m / z 528.2435[M+H] + ;calcd for C 30 h 31 FN 5 o 3 + [M+H] + 528.2405.
Embodiment 3
[0057] N-(6-((2-aminophenyl)amino)-6-oxohexyl)-2-fluoro-5-((4-oxo-3,4-dihydro-phthalazin-1-yl) Methyl) benzamide (compound 3)
[0058]
[0059] According to the preparation method of compound 1, 4-(aminomethyl)benzoic acid was replaced with 6-aminocaproic acid, and other operations were the same. The product is brown-yellow solid. m.p.104℃-106℃. 1 H NMR (600MHz, DMSO-d 6 )δ (ppm): 12.61 (s, 1H), 9.11 (s, 1H), 8.35–8.18 (m, 2H), 7.95 (d, J = 8.0Hz, 1H), 7.87 (t, J = 7.2Hz, 1H), 7.81(t, J=7.3Hz, 1H), 7.51(t, J=10.9Hz, 1H), 7.42(s, 1H), 7.14(dt, J=35.8, 18.7Hz, 2H), 6.85( t,J=7.4Hz,1H),6.68(d,J=7.7Hz,1H),6.49(t,J=7.0Hz,1H),4.30(s,2H),3.20(d,J=5.5Hz, 2H), 2.29(t, J=7.0Hz, 2H), 1.58(d, J=6.8Hz, 2H), 1.50(s, 2H), 1.32(s, 2H).HRMS(ESI-TOF): m / z 502.2269[M+H] + ;calcd for C 28 h 29 FN 5 o 3 + [M+H] + 502.2249.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap