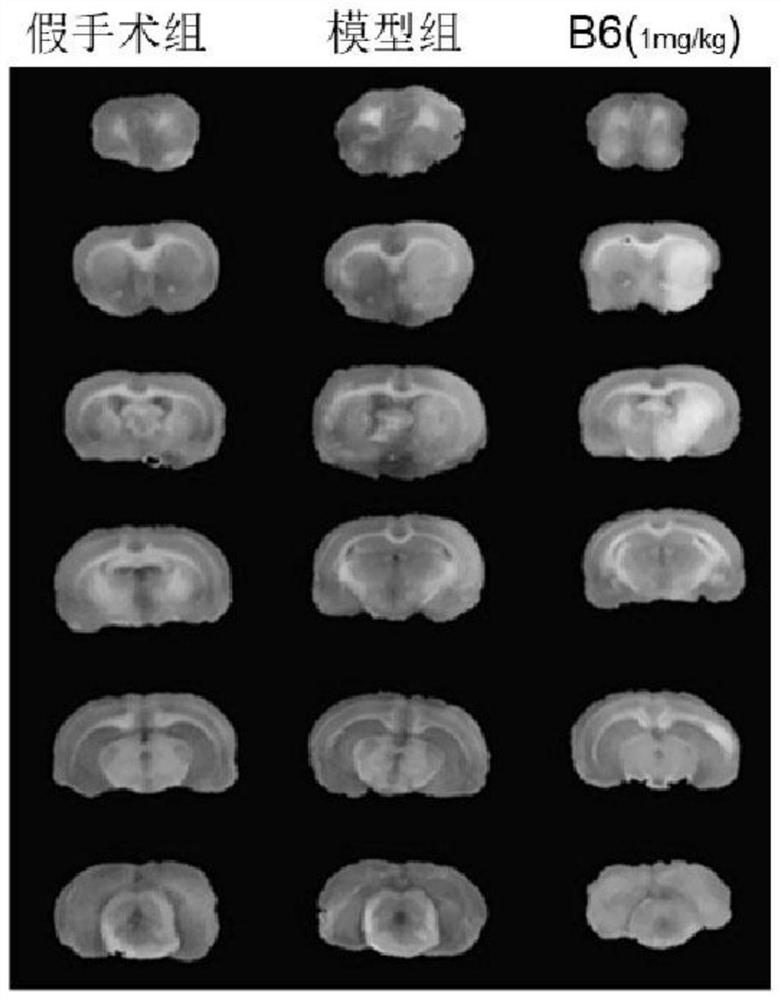
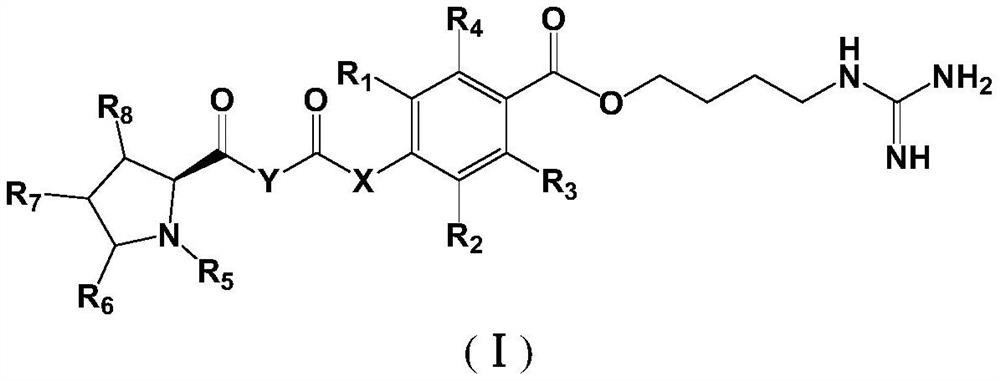
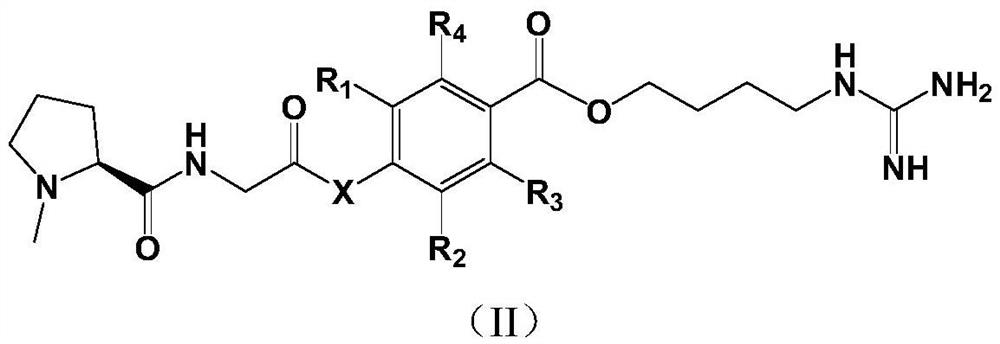
Proline derivatives and their application in the preparation of drugs for treating cardiovascular and cerebrovascular diseases
A technology for cardiovascular and cerebrovascular diseases and derivatives, applied in the field of medicine, can solve the problems of low bioavailability, short half-life of stachydrine, poor anti-ischemic stroke activity, etc., and achieve the effect of inhibiting cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: the synthetic route of N-methyl-L-proline b is as follows:
[0049]
[0050] Dissolve L-proline (4.0 g, 34.8 mmol) in methanol (40 mL), add 40% aqueous formaldehyde solution (2.8 mL, 38.2 mmol) and 10% palladium on carbon (1 g) in turn, add hydrogenation, at room temperature The reaction was carried out for 24 hours. After the reaction was completed, it was filtered, and the mother liquor was concentrated to dryness to obtain 4 g of white solid with a yield of 89%, which was N-methyl-L-proline (compound b). ESI-MS: (m / z, %)=130 [M+H] + .
Embodiment 2
[0051] Embodiment 2: The synthetic route of compound B-4 is as follows:
[0052]
[0053] The N-methyl-L-proline b (1.29 g, 10 mmol) was added to dichloromethane (20 mL), followed by DCC (2.47 g, 12 mmol) and glycine (0.75 g, 10 mmol), and reacted at room temperature for 24 hours, filtered, and concentrated the mother liquor to obtain 1 g of white solid with a yield of 54%, which was compound B-4. 1 H NMR (500MHz, CD 3 OD)δ=4.14(t,J=8.3,1H),4.00(s,2H),3.71(ddd,J=11.4,7.7,4.1,1H),3.22(d,J=11.1,1H),2.95( d, J=10.5, 3H), 2.58 (dd, J=12.2, 7.5, 1H), 2.24-2.02 (m, 3H).
Embodiment 3
[0054] Embodiment 3: the synthetic route of compound B-5 is as follows:
[0055]
[0056] The B-4 (1.86 g, 10 mmol) was added to dichloromethane (20 mL), followed by DCC (2.47 g, 12 mmol) and Y5 (3.1 g, 10 mmol), reacted at room temperature for 24 hours, filtered, and the mother liquor was concentrated to obtain no residue. The color oily substance was 1.4 g, the yield was 30%, which was compound B-5. ESI-MS: (m / z, %)=480 [M+H] + . 1 H NMR (500MHz, D 2 O)δ=7.43(s, 2H), 4.49(d, J=7.8Hz, 2H), 4.42(t, J=6.5Hz, 2H), 4.31(t, J=8.5Hz, 1H), 3.92(s) ,6H),3.85-3.80(m,1H),3.31-3.25(m,3H),2.99(s,3H),2.68-2.61(m,1H),2.32-2.04(m,3H),1.94-1.84 (m, 2H), 1.80–1.73 (m, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com