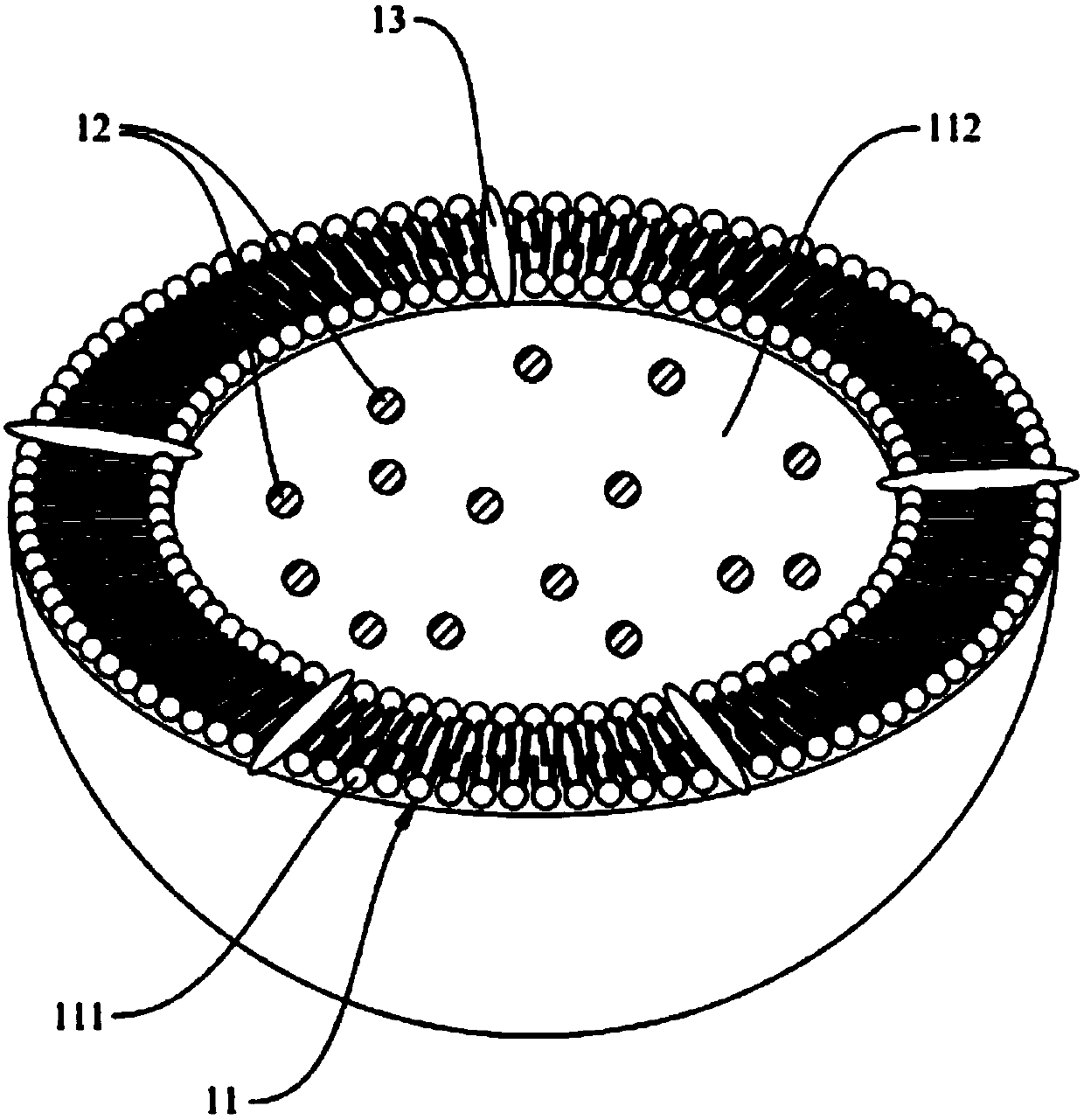
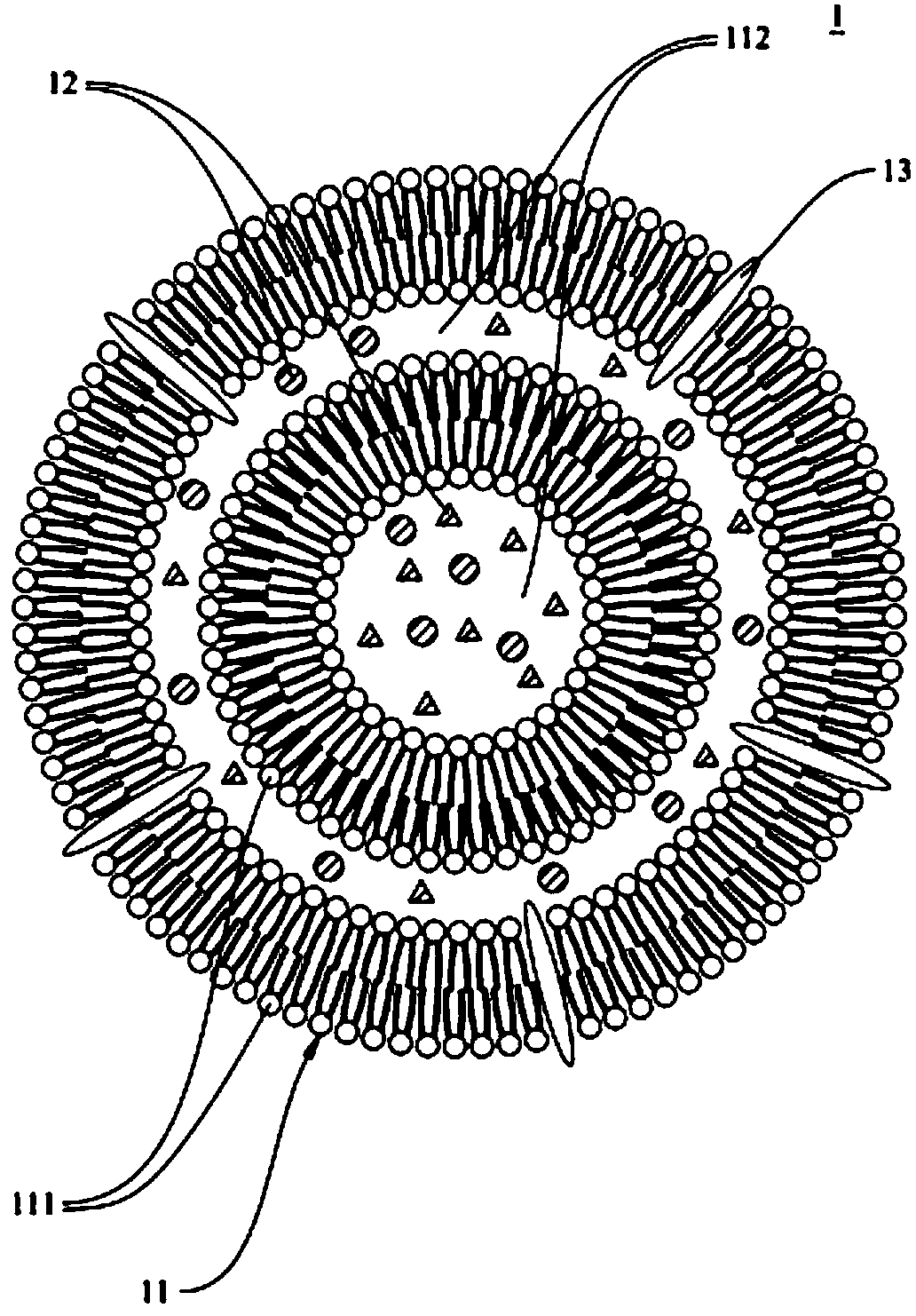
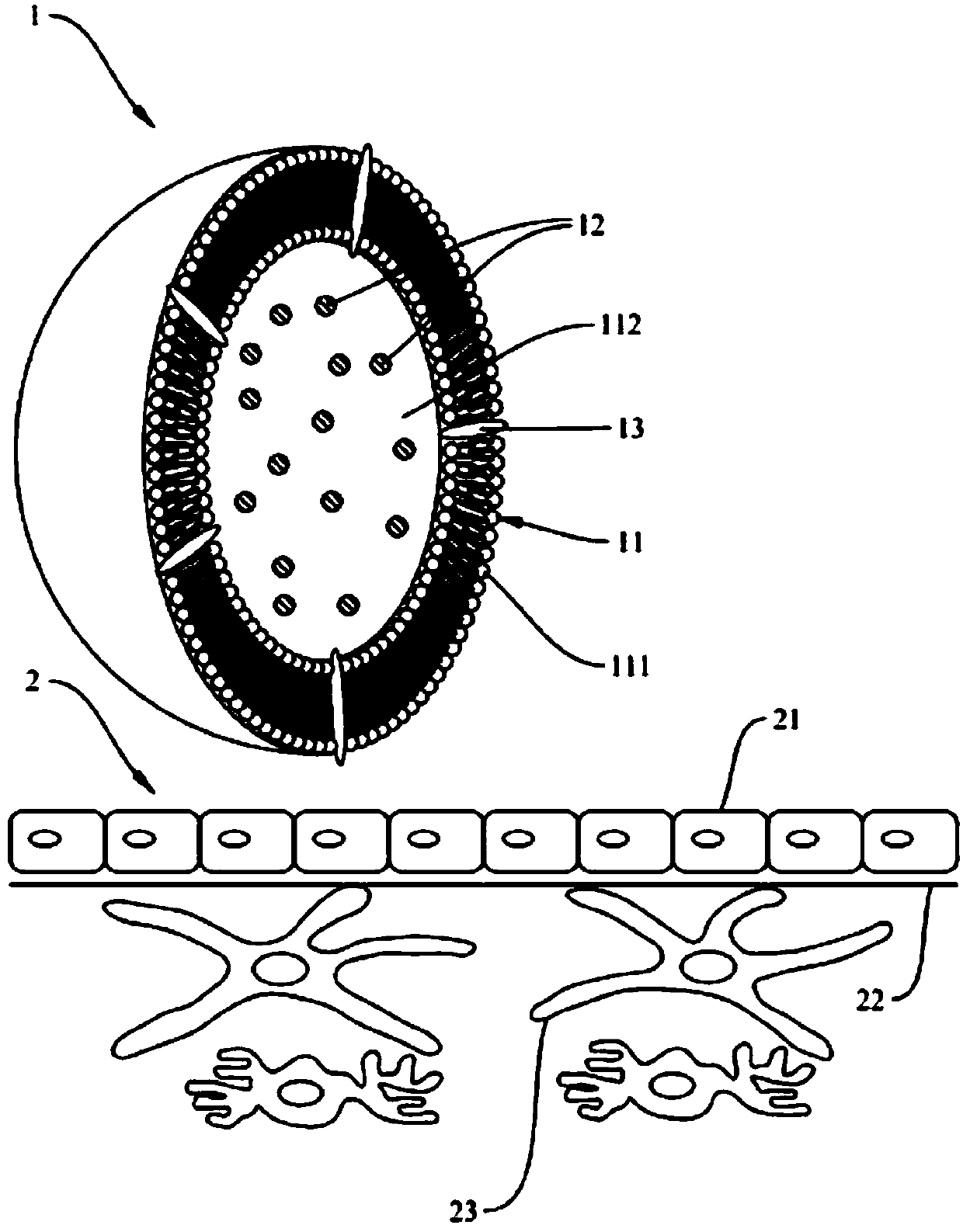
Microliposome composition
A composition and phospholipid technology, applied in the directions of drug combination, liposome delivery, medical preparations of inactive ingredients, etc., can solve the problems of difficult and difficult treatment of brain diseases, and achieve the regulation of neuroplasticity and the promotion of synapses. Generate effect, enhance the effect of loop
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] [Example 1]-Mobility test of administration liposome composition
[0076] Four first-generation mice born in the same littermate and raised in the animal room for 8 months were subjected to a preliminary cylinder test, in which a wild-type mouse of a healthy model and a transgenic mouse of a disease model were administered with the mouse of Comparative Example 1 Mixed solution, the other two transgenic mice of the disease model were administered with Embodiment 1. Three tests were performed per mouse.
[0077] refer to Figure 5 , which shows the average number of forelimb-wall contacts and error bars for these mice within five minutes. In the mouse group administered with Comparative Example 1, the activity of the disease model mice was significantly lower than that of the littermate healthy model mice (P=0.018). The activity of the disease model mice administered with Embodiment 1 was significantly higher than that of the disease model mice administered with Compar...
Embodiment 2
[0078] [Example 2]-administration of activity tests containing liposome compositions with different nutrients
[0079] Two first-generation mice born in the same littermate and raised in the animal room for 8 months were subjected to a preliminary cylinder test. One of the transgenic mice of the disease model was administered with Comparative Example 2, and the other transgenic mouse of the disease model was administered with Implementation Pattern 1. Three tests were performed per mouse.
[0080] The results are shown in Table 1 below. The activity of the disease model mice administered with Embodiment 1 was higher than that of the disease model mice administered with Comparative Example 2. In other words, among the mice that are also disease models, the PD symptoms (increased activity) of the mice administered with Embodiment 1 were significantly improved (increased in activity) compared with the mice administered with Comparative Example 2. Therefore, the liposome composit...
Embodiment 3
[0083] [Example 3] - Survival analysis
[0084] In order to test the lethal side effects of the present invention on mice, in Example 3, the disease model mice and the healthy model mice in the same litter were fed with Embodiment 1 and Comparative Example 1 respectively.
[0085] refer to Image 6 , which shows the survival analysis results. The results showed that all other healthy and disease models administered with Embodiment 1 or Comparative Example 1 died after 10 weeks of continuous treatment with Embodiment 1 (10 mg / day for two consecutive weeks) except one disease model mouse. All mice survived, and no immediate treatment-related death, nor apparent abnormal behavior was observed in the surviving mice. This result indicated that administration of Embodiment 1 for continuous treatment (10 mg / day for two consecutive weeks) did not result in immediate death of the mice.
[0086] In summary, the double-layer phospholipid layer and the special targeting substance of th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com