Polyketide indole alkaloid and its preparation method and application
An alkaloid, indole polyketone technology, applied in the field of microbial engineering technology and pharmacology, can solve the problems of restricting the treatment effect and quality of life of tumor patients, drug resistance, low treatment efficiency, etc. Guaranteed, low-cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: Liquid fermentation and feeding of mantis intestinal bacteria IFB-T01 (Daldinia eschscholzii)
[0032] Daldinia eschscholzii IFB-TL01, which is derived from the intestinal bacteria of the activated mantis mantis, was inoculated into 1L Erlenmeyer flasks, each containing 400mL of malt culture medium, inoculated into 10 flasks on a shaker, and cultured at 200rpm, 28-30℃ for 2 ~3 days, as the seed solution, and then inoculate the seed solution in a new malt culture medium (400mL / bottle×200 bottles) with an inoculation amount of 20mL, and continue to cultivate for 2 days at 200rpm and 28 to 30°C. Feed indole-3-carbinol at 24, 48, and 72 hours respectively until the final concentration of indole-3-carbinol in the solution is 1.0 mM, 200 rpm, and then continue fermentation at 28-30°C for 10 days.
Embodiment 2
[0033] Embodiment 2: Extraction and separation of indole polyketide alkaloids
[0034] Get the fermented liquid in Example 1 and filter it through gauze, extract the filtrate with ethyl acetate, and centrifuge and concentrate to obtain black extract A (83g); extract A is subjected to silica gel column chromatography segmentation, and petroleum ether: acetone (volume ratio: 100:2, 100:5, 10:1, 5:1, 3:1, 2:1, 1:1) for gradient elution to obtain 7 eluted fractions A1-A7;
[0035] Section A4 (petroleum ether: acetone = 5:1 part) continued to be eluted with petroleum ether: acetone (100:2→1:1) gradient to obtain a total of 7 fractions F1-F7.
[0036] Among them, F3 (20:1) and F4 (10:1) fractions were subjected to Sephadex LH-20 column chromatography (methanol elution), and high-pressure preparative liquid phase [chromatographic column: ODS-2Hy0persil columns (5 μm, 250 × 10mm) ], MeOH / H 2 O (70:30 and 65:35) elution purification, respectively to obtain the racemate (8mg, yield ...
Embodiment 3
[0038] Embodiment 3: Structural identification of indole polyketide alkaloids
[0039] The structures of indolepolyketone alkaloids were determined based on their mass spectrum, nuclear magnetic resonance spectrum, and X-ray single crystal diffraction analysis.
[0040] The spectroscopic data are as follows:
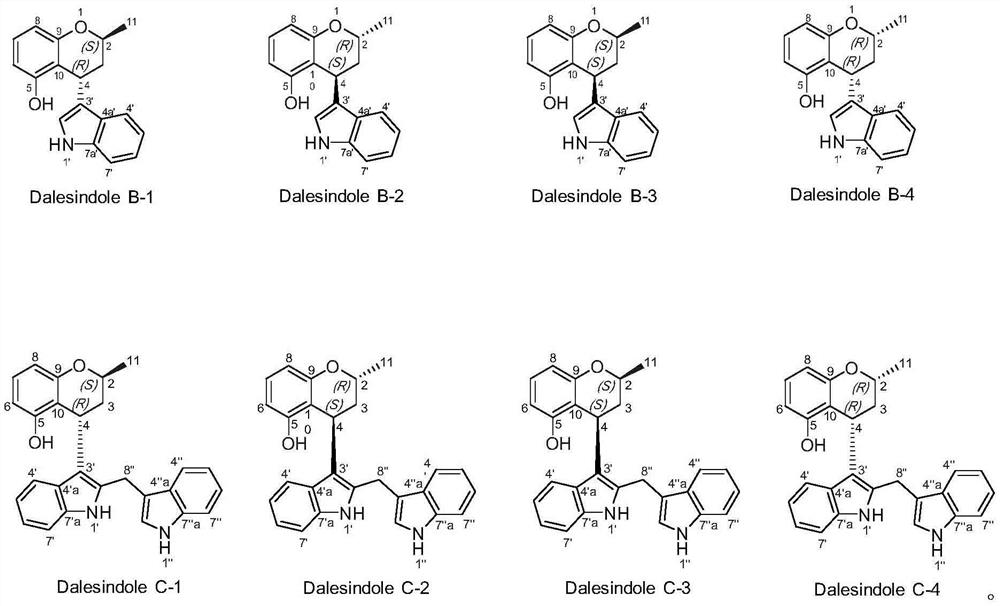
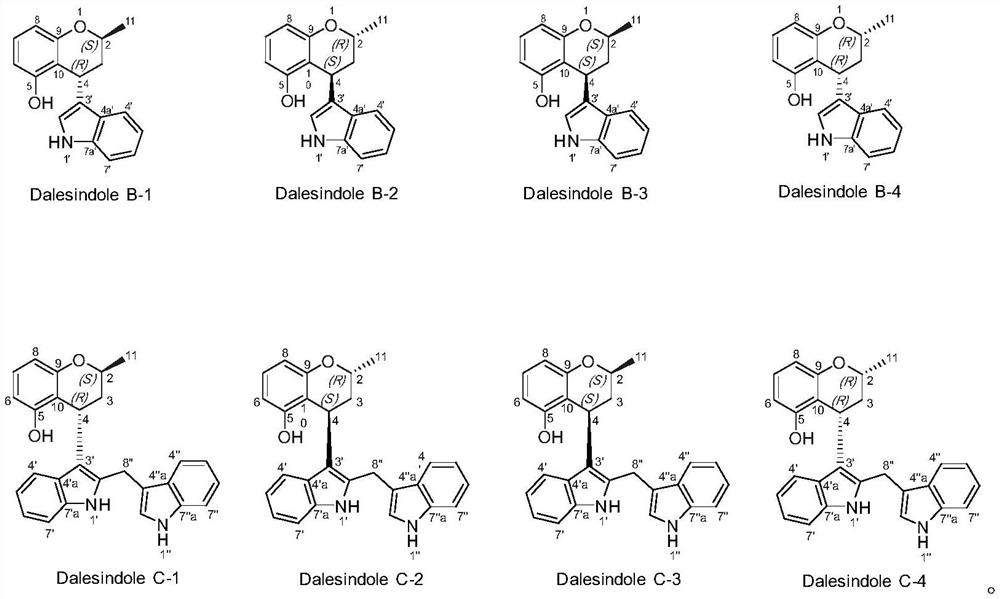
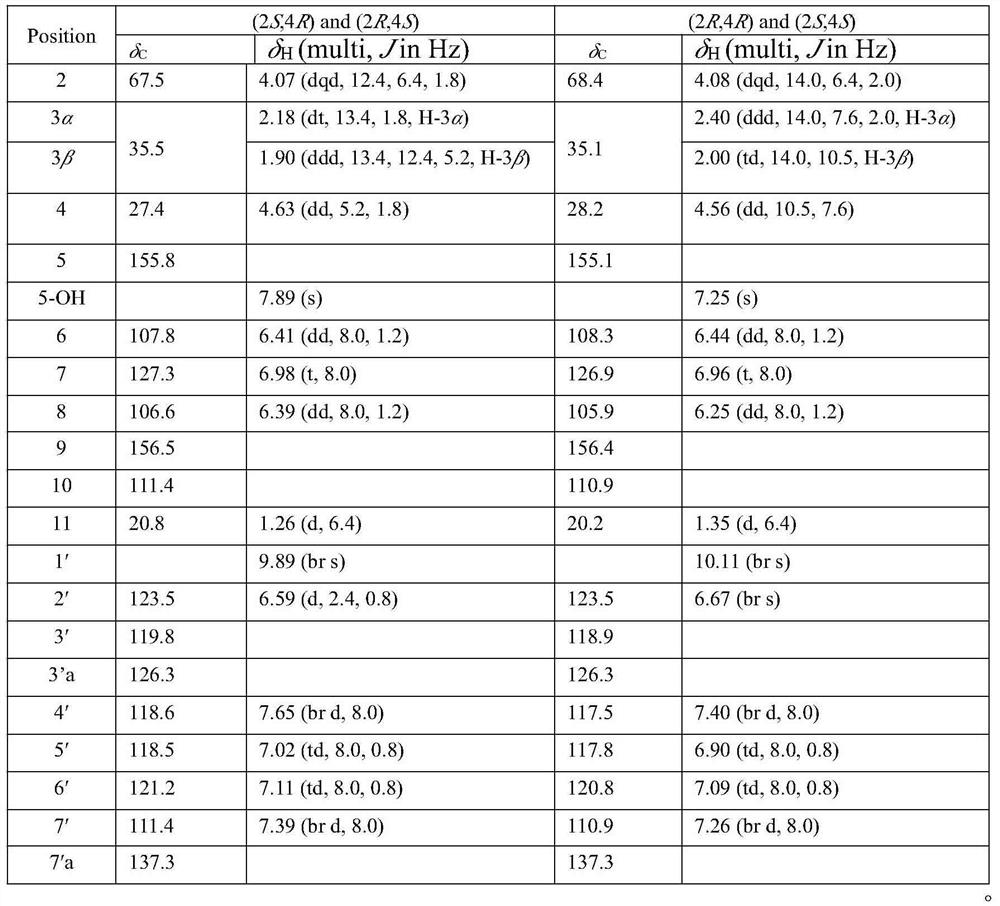
[0041] Dalesindole B, from CH 2 Cl 2 / MeOH (1:1) yellow single crystal, high resolution electrospray mass spectrometry HR / ESIMS: m / z 302.1156; infrared (KBr)ν max (cm -1 ):3409,2971,2913,1616,1587,1464,744. 1 H and 13 C NMR is shown in Table 1.
[0042] Chiral resolution of racemate A obtained two enantiomers {(+)-(2S,4R), light gray powder, high resolution electrospray mass spectrometry HR / ESIMS: m / z302.1159; optical rotation [α] 25D+25.3°(c=0.15, MeOH), circular dichroism CD(MeOH)λ max (Δε)=204(5.5), 228(-6.1), 270(1.38)nm; (–)-(2R,4S), light gray powder, high resolution electrospray mass spectrometry HR / ESIMS: m / z 302.1154; optical rotation [α]25D–20.0°(c=0.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com