Organometallic iridium complex and simple synthesis method and uses thereof
An iridium complex, organometallic technology, applied in indium organic compounds, platinum group organic compounds, organic chemistry, etc., can solve the problems of undisclosed coordination compound synthesis, undisclosed organometallic iridium complex synthesis methods, etc., Achieve the effect of improving synthesis yield, high quantum efficiency, and inhibiting the degree of attenuation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] First, in step (a1), 10 millimoles (mmol) of 4-tert-butylphenylbromide (4-tert-butylphenylbromide) is dissolved in 20 milliliters of THF to form a solution, and the solution is formed under nitrogen atmosphere (N 2 ), freshly dried magnesium particles (11 mmol) were added to the aforementioned solution, and after half an hour of reaction, the Grignard reagent (Grignard reagent) of 4-tert-butylphenylmagnesium bromide was obtained.
[0053] In step (a2), 10 mmol of phenyl chloroformate (phenyl chloroformate) and 10 mmol of 4-tert-butylpyridine (4-tert-butylpyridine) were reacted in 20 ml of anhydrous THF at -20°C for half an hour , to form nitrogen-containing heteroaromatic ring salts of 4-tert-butylpyridinium chloride.
[0054] In step (a3), the Grignard reagent of 4-tert-butylphenylmagnesium bromide is slowly added into 20 ml of THF solution containing the nitrogen-containing heteroaromatic ring salts by syringe injection, and slowly heated The reaction solution was st...
Embodiment 2
[0068] Coordination compound 2 is synthesized from steps (a1) to (a3) and step (b) similar to the synthesis of coordination compound 1, the difference is that 6-tert-butylquinoline in step (a2) replaces 4-tert Butylpyridine; in step (b) use a mixture of n-hexane and ethyl acetate (volume ratio of 16:1) to purify the crude product, its yield is 65%, and the mixed solution of dichloromethane and hexane After purification by recrystallization, white crystals were obtained, namely coordination compound 2.
[0069] The chemical structure identification of the coordination compound 2 is carried out with a nuclear magnetic resonance spectrometer, and its characteristic peaks are as follows: 1 H-NMR (CDCl 3 ): δ8.17 (d, 1H, J = 8.7Hz, quinoline characteristic peak), 8.13 (d, 1H, J = 9.0Hz, quinoline characteristic peak), 8.10 (d, 2H, J = 8.4Hz, benzene ring characteristic peak), 7.84(d,1H,J=8.7Hz, quinoline characteristic peak), 7.83(dd,1H,J 1 =9.0Hz,J 2 =2.1Hz, characteristic p...
Embodiment 3
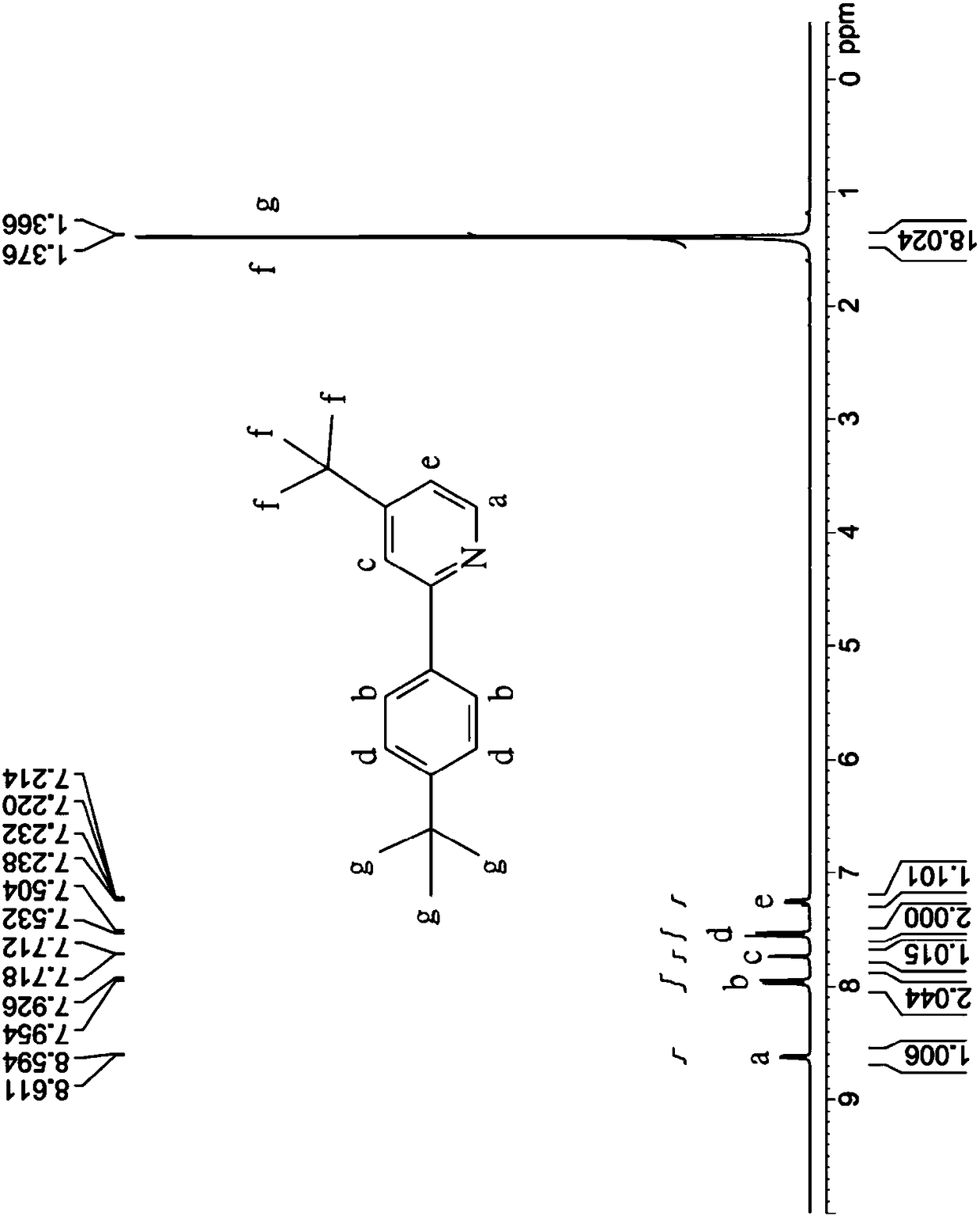
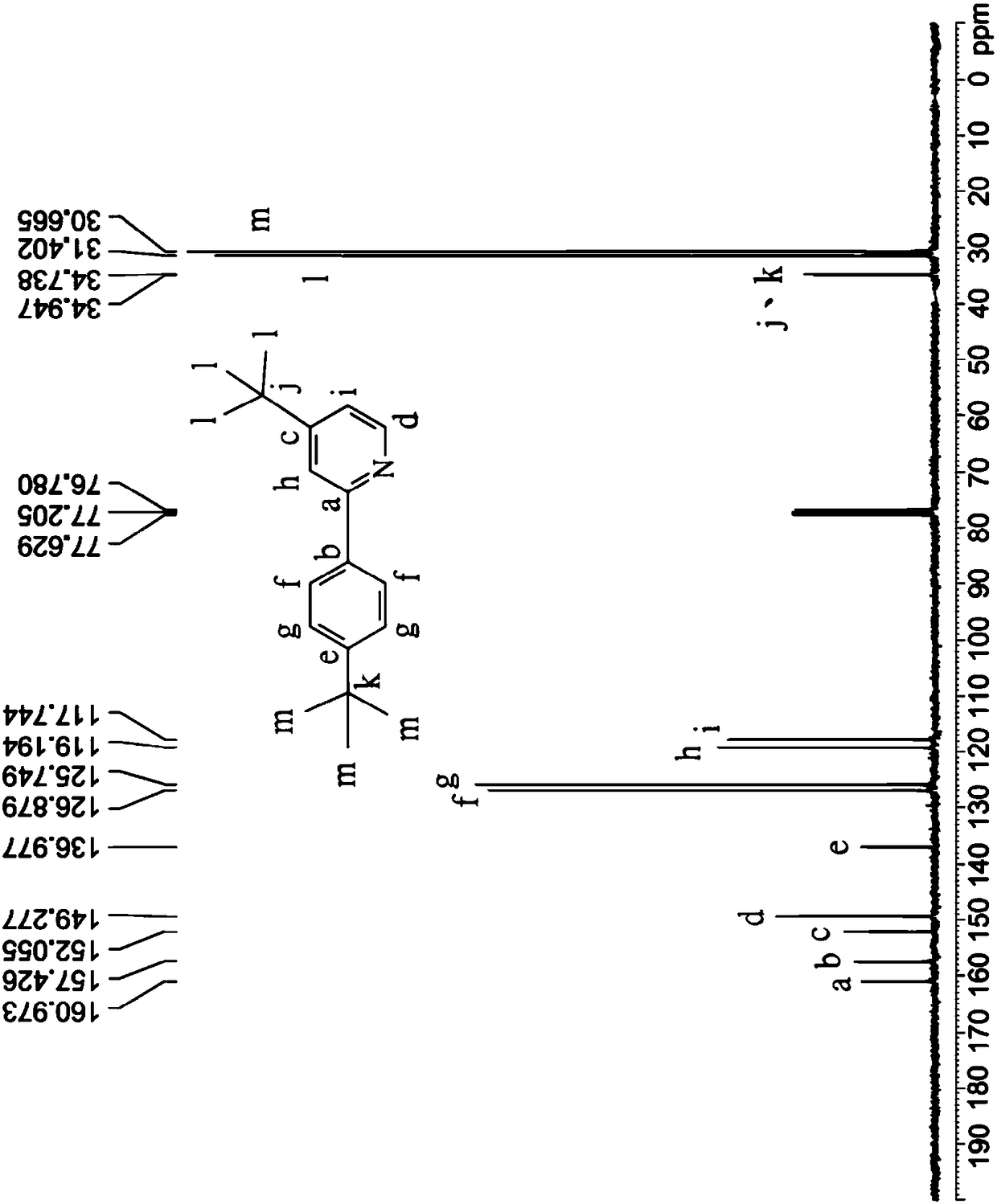
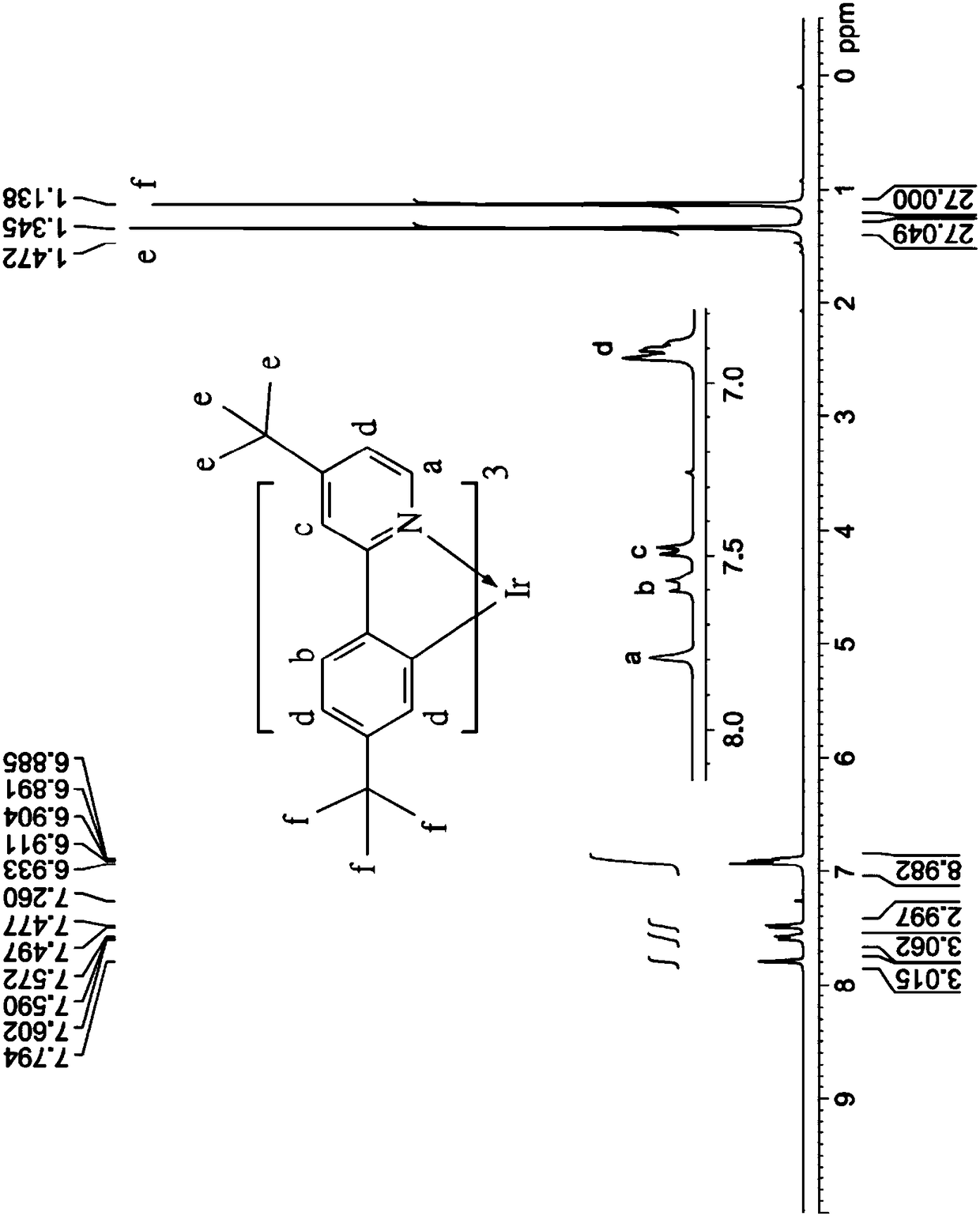
[0075] Coordination compound 3 is synthesized similarly to step (a1) to step (a3) and step (b) of the synthesis of coordination compound 1, the difference is that the starting material of step (a1) is 4-bromo-4'- (tert-butyl)-1,1'-biphenyl substituted 4-tert-butylbromobenzene; in step (b) the crude The product, the yield of which was 70%, was purified by recrystallization from a mixed solution of dichloromethane and methanol to obtain white crystals, namely coordination compound 3.
[0076] The chemical structure identification of the coordination compound 3 is carried out with a nuclear magnetic resonance spectrometer, and its characteristic peaks are as follows: 1 H-NMR (CDCl 3 ): δ8.62 (d, 1H, J = 5.1Hz, characteristic peak of pyridine), 8.06 (d, 2H, J = 8.4Hz, characteristic peak of benzene ring), 7.76 (d, 1H, J = 1.2Hz, characteristic peak of pyridine peak), 7.72(d,2H, J=8.4Hz, characteristic peak of benzene ring), 7.63(d,2H, J=8.7Hz, characteristic peak of benzene ri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com