First-class phenylmethylamine triazine structural COMT inhibitor, as well as preparation method and application thereof
A C1-C5 compound technology, applied in the field of COMT inhibitors, can solve problems such as limited applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
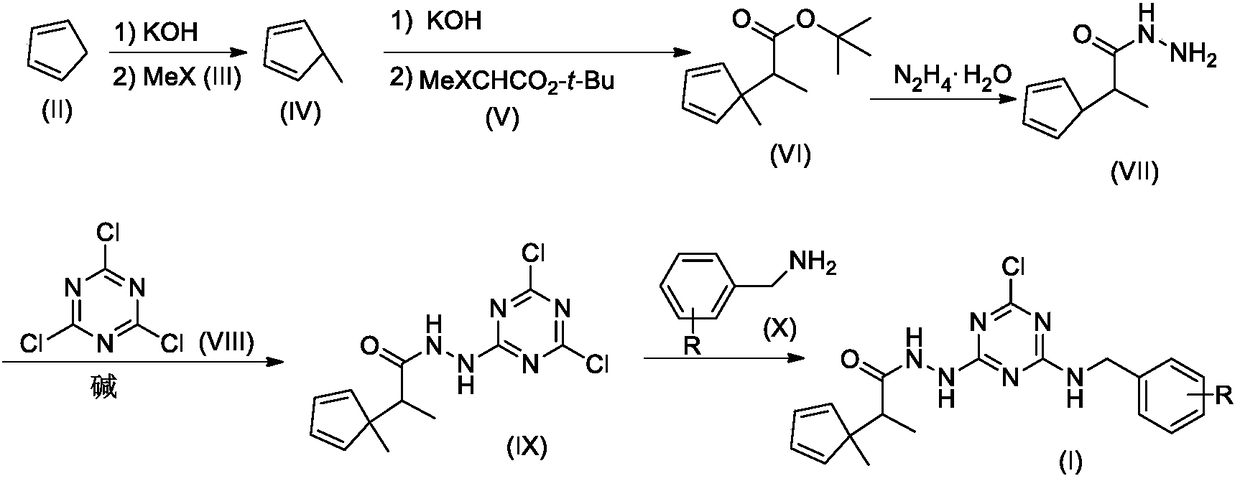
[0020] Example 1 Synthesis of Compound I-1
[0021]
[0022] Step 1. Synthesis of compound VI-1
[0023] Compound II (1.32g, 20mmol) was dissolved in 20mL DMSO, stirred at room temperature, KOH solid (2.24g, 40mmol) was added, and stirring was continued for 1 hour at room temperature. Then MeI (III-1,2.84g, 20mmol) was added, and stirring was continued overnight at room temperature. Then, tert-butyl bromoacetate V-1 (3.90 g, 20 mmol) was added, and stirring was continued for 12 hours. TLC detection revealed that the reaction was complete.
[0024] The reaction mixture was carefully poured into 200mL ice water, stirred, and 50mL×3CH 2 Cl 2 After extraction, the extracts were combined, washed with 100 mL of 5% brine, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filtration, the filtrate was evaporated to dryness on a rotary evaporator, and the residue was purified by silica gel column chromatography to obtain compound VI-I, 2.99 g (combined yield 77%)....
Embodiment 2
[0031] Example 2 Synthesis of Compound I-2
[0032]
[0033] Step 1. Synthesis of compound VI-2
[0034] Compound II (1.32g, 20mmol) was dissolved in 20mL DMSO, stirred at room temperature, KOH solid (2.24g, 40mmol) was added, and stirring was continued for 1 hour at room temperature. Then MeI (III-1,2.84g, 20mmol) was added, and stirring was continued overnight at room temperature. Then 20mmol V-2 was added and stirring was continued for 12 hours. TLC detection revealed that the reaction was complete. The reaction mixture was carefully poured into 200mL ice water, stirred, and 50mL×3CH 2 Cl 2 After extraction, the extracts were combined, washed with 100 mL of 5% brine, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filtration, the filtrate was evaporated to dryness on a rotary evaporator, and the residue was purified by silica gel column chromatography to obtain compound VI-2. ESI-MS, m / z=209([M+H] + ).
[0035] Step 2. Synthesis of compound VII-2...
Embodiment 3-5
[0040] With reference to the methods of Examples 1 and 2, the following compounds were synthesized:
[0041]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com