Monocarboxylate transport modulators and uses thereof
一种选自、药学的技术,应用在单羧酸转运蛋白调节剂及其用途领域,能够解决没有应用癌症治疗等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
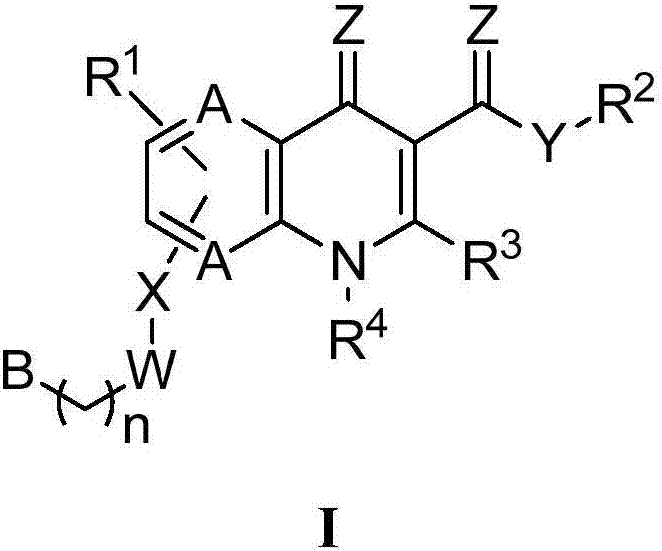
Image
Examples
Embodiment 1
[0313]
[0314] step 1
[0315]
[0316] method
[0317] In a 250 mL 3-necked round bottom flask, 3-chloro-4-fluoroaniline (20 g, 137.4 mmol) and toluene (100 mL) were added. To the reaction mixture was added diethyl-2-(ethoxymethylene)malonate (29.7 g, 137.4 mmol) at 25°C. The reaction mixture was heated at reflux and stirred for 1 h, then cooled to 30 °C and water (150 mL) was added and the mixture was extracted with EtOAc (30 mL x 3). The combined organic layers were washed with brine solution, washed with anhydrous Na 2 SO 4 Drying and concentration under reduced pressure afforded 40 g of crude product which was titrated with a minimal amount of cyclohexane to give diethyl-2-(3-chloro-4-fluorophenylamino)methylenemalonate (1) 36g, yield (50.1%); 1 H NMR (400MHz, DMSO-d 6): δ10.61(d, J=12.8,1H),8.29(d,J=13.6,1H),7.72-7.74(m,1H),7.4-7.42(m,2H),4.12-4.2(m, 4H), 1.24-1.26 (m, 6H); MS (ESI): 316.1 (M+H).
[0318] step 2
[0319]
[0320] In a 500 mL 3-neck rou...
Embodiment 2
[0331] The remaining mother liquor from the synthesis of Example 1 was concentrated under reduced pressure to obtain crude 5b. The product was purified by column chromatography (silica gel) to obtain 10 mg of pure 6-(benzyl(methyl)amino)-7-chloro-1-isobutyl-4-oxo-1,4-dihydroquinoline- 3-Carboxylic acid (5b): yield, 5%; 1 H NMR (400MHz, DMSO-d 6 ):δ15.16(d,1H),8.95(s,1H),7.28-8.27(m,7H),4.41-4.43(d,2H),4.3(s,2H),2.74(s,3H), 2.1-2.2 (m, 1H), 0.88-0.90 (d, 6H); MS (ESI): 399 (M+H); HPLC: 93.57%.
Embodiment 3
[0333]
[0334] In a 25 mL single necked round bottom flask were added intermediate 4 (0.5 g, 1.679 mmol) from Example 1 and N-1-(3-(trifluoromethyl)phenyl)methanamine (1.5 g, 8.39 mmol) ) and heated to 120°C for 24h. The reaction was monitored for completion by TLC, worked up aqueous and acidified to pH 3-4 with 1 N HCl. The aqueous layer was extracted with EtOAc and the organic layer was washed with anhydrous Na 2 SO 4 dry. The solvent was evaporated under reduced pressure to obtain a crude product which was purified by silica gel column chromatography to obtain pure 6-fluoro-1-isobutyl-7-(methyl(3-(trifluoromethyl)benzyl) Amino)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (5), (25mg); Yield: 3.3%; 1 H NMR (400MHz, DMSO-d 6 ):δ15.5(s,1H),6.83-8.71(m,7H),4.75(s,2H),4.20(d,2H),3.13(s,3H),1.83-1.86(m,1H), 0.74-0.76 (d,6H); MS (ESI): 451.1 (M+H); HPLC: 92.07%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com