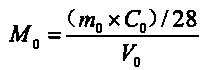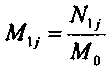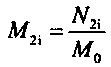A method for dividing reaction kinetics stages in fly ash strong alkali system
A technology of reaction kinetics and fly ash, applied in chemical method analysis, scientific instruments, analytical materials, etc., can solve problems that hinder basic theory and practical application research, affect the deepening and expansion of fly ash application fields, and achieve easy The effect of the operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1) Strong base system reaction
[0031] The fly ash sample was reacted with a strong alkali solution (5mol / L NaOH solution) according to the solid-to-liquid ratio (mass ratio: 1 / 10), the reaction temperature was 35°C, and the reaction time was 0-48h.
[0032] 2) Calculate the original silicon parameter M 0
[0033] Calculate the apparent substance concentration of silicon in the reaction system by formula (1), and record it as the original silicon parameter M 0 , its value is 2.3mol / L.
[0034]
[0035] where m 0 (g) is the quality of fly ash in the reaction system, C 0 (%) is the mass fraction of silicon in fly ash, V 0 (L) is the volume of the mixed system.
[0036] 3) Silicon parameter series 1
[0037] After the strong alkali system reaction is completed, filter the mixed solution through a 0.45 μm filter membrane to detect the silicon element concentration in the solution, and obtain the silicon parameter series 1, which is recorded as [N 1j ].
[0038]...
Embodiment 2
[0055] Method is the same as embodiment 1, wherein,
[0056] 1) Strong base system reaction
[0057] The fly ash sample was reacted with a strong alkali solution (7.5mol / L NaOH solution) according to the solid-to-liquid ratio (mass ratio: 1 / 20), the reaction temperature was 25°C, and the reaction time was 0-48h.
[0058] 2) Calculate the original silicon parameter M 0
[0059] Calculate the apparent substance concentration of silicon in the reaction system by formula (1), and record it as the original silicon parameter M 0 , and its value is 1.15mol / L.
[0060] 3) Silicon parameter series 1
[0061] After the strong alkali system reaction is completed, filter the mixed solution through a 0.45 μm filter membrane to detect the silicon element concentration in the solution, and obtain the silicon parameter series 1, which is recorded as [N 1j ].
[0062] 4) Silicon parameter series 2
[0063] Use a dilute mixed acid solution (30% HF, 30% HCl and 40% citric acid) to react wit...
Embodiment 3
[0076] Method is the same as embodiment 1, wherein,
[0077] 1) Strong base system reaction
[0078] The fly ash sample was reacted with a strong alkali solution (10mol / L NaOH solution) according to the solid-to-liquid ratio (mass ratio: 140), the reaction temperature was 50°C, and the reaction time was 0-48h.
[0079] 2) Calculate the original silicon parameter M 0
[0080] Calculate the apparent substance concentration of silicon in the reaction system by formula (1), and record it as the original silicon parameter M 0 , and its value is 0.575mol / L.
[0081] 3) Silicon parameter series 1
[0082] After the strong alkali system reaction is completed, filter the mixed solution through a 0.45 μm filter membrane to detect the silicon element concentration in the solution, and obtain the silicon parameter series 1, which is recorded as [N 1j ].
[0083] 4) Silicon parameter series 2
[0084] Use dilute mixed acid solution (30% HF, 30% HCl and 40% citric acid) to react with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com