Application of dalbavancin in the preparation of drugs for treating AIDS
A technology for dalbavancin and AIDS, applied in antiviral agents, instruments, glycopeptide components, etc., can solve the problem of the anti-HIV activity of dalbavancin, which has not been seen, and the rapid and accurate prediction and discovery of anti-HIV Active methods and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
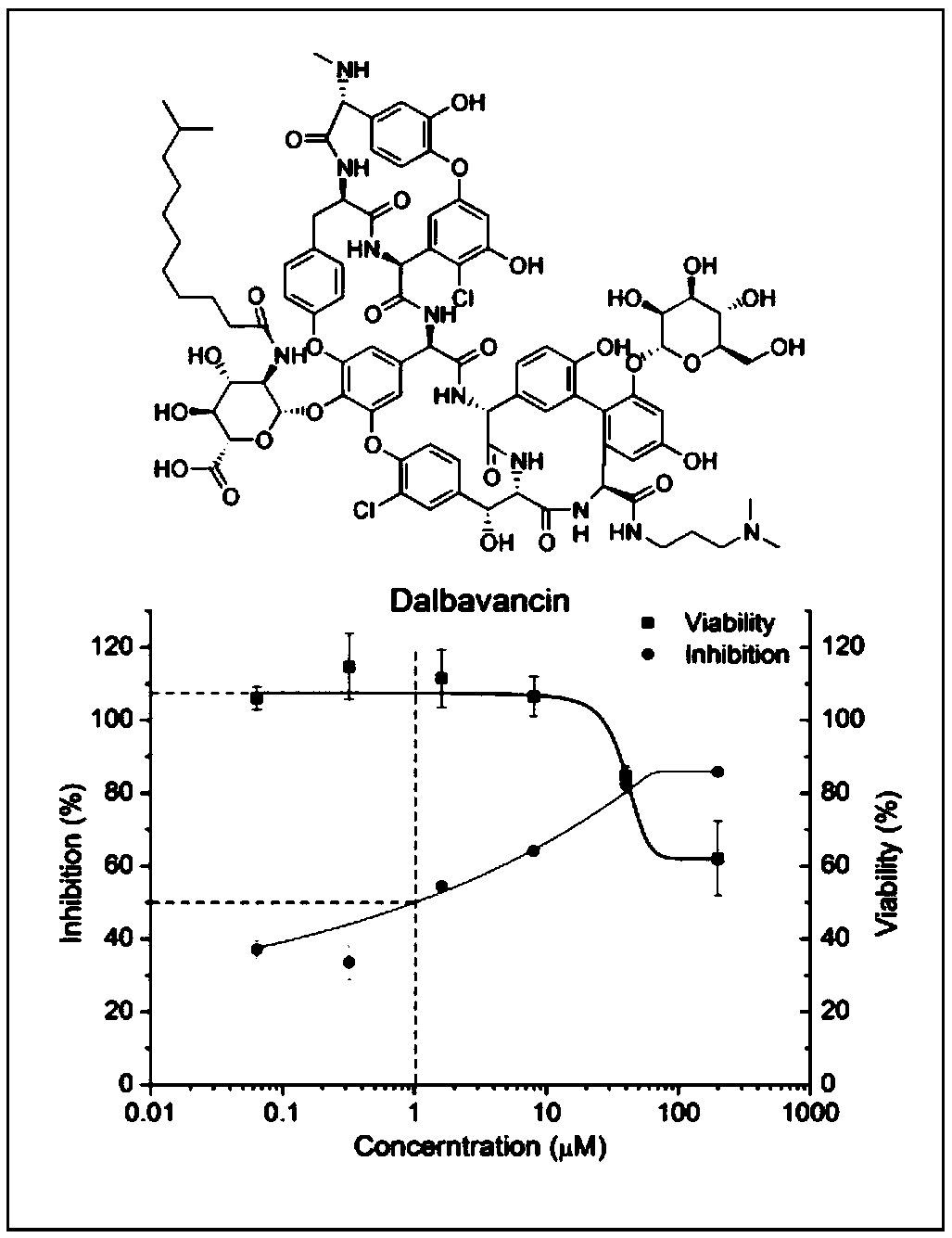
[0025] Testing the anti-HIV effects of Dalbavancin:
[0026] 1. Purpose of the experiment: To test the anti-HIV effect of Dalbavancin
[0027] 2. Experimental materials
[0028] 2.1. Determination of Drugs and Compounds
[0029] Dalbavancin was purchased from Beijing Lebo Biotechnology Co., Ltd. The positive control compound azidothymidine (3'-Azido-3'-deoxythymidine, AZT) was purchased from Sigma. The sample to be tested is dissolved in RPMI-1640 complete medium or DMSO according to solubility, the sample stock solution concentration dissolved in DMSO is 50mM, and the sample stock solution concentration dissolved in RPMI-1640 complete medium is 10mM, 5mM or 2mM ( According to the solubility), the storage conditions are: -20°C; AZT is dissolved in RPMI-1640 complete medium, sterilized by filtration with a 0.22 μm filter membrane, the concentration of the stock solution is 6 mg / ml, and stored at -20°C after aliquoting.
[0030] 2.2. Reagents and solutions
[0031] 2.2.1. R...
Embodiment 2
[0061] Add excipients according to the weight ratio of dalbavancin (Dalbavancin) to excipients 1:1 or 1:2, granulate and compress into tablets.
Embodiment 3
[0063] Dalbavancin (Dalbavancin) is an active ingredient, and it is made into capsules according to a conventional capsule preparation method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com