Artesunate derivatives, preparation methods and applications thereof
A technology of artesunate and reaction, applied in the directions of drug combination, skin diseases, bone diseases, etc., can solve the problems such as inability to fundamentally control the occurrence and development of diseases, incapable of long-term continuous application, large toxic and side effects, etc. Down-regulation of cellular immune response, simple and easy preparation method, and little effect of environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]
[0038] Weigh 100g of artesunate (ART) into a 2L round bottom flask, add 47.9g of N-hydroxysuccinimide (NHS) and 80g of EDC hydrochloride (EDC.HCl), add 1200ml of acetonitrile, control Warm at 30°C and react overnight with stirring. Spin-dry acetonitrile under reduced pressure, add 1000ml ethyl acetate (EA) to dissolve, wash with 500ml water three times each time, wash once with 500ml saturated sodium chloride, and dry the organic phase with anhydrous sodium sulfate for 1h.
[0039] Filter through a Buchner funnel, evaporate EA under reduced pressure to about 250ml, heat the solution to reflux, slowly add 140ml of petroleum ether until the precipitate just precipitates, and put it in the refrigerator (4°C) overnight to precipitate crystals.
[0040] Filter with a G3 funnel, wash the solid twice with 150 ml of anhydrous diethyl ether, and pump dry to obtain 113 g of solid artesunate succinimide ester (ART-NHS) crude product, which is directly used for the next reacti...
Embodiment 2
[0042]
[0043] Put 14.5g of LYS-OH in a 2L round bottom flask, add 1000ml of methanol (MeOH), stir for 1h, add 100g of ART-NHS crude product prepared in Example 1, add N, N diisopropylethylamine (DIEA) 17.3 ml, control the internal temperature to 35°C, and react for 8 hours under stirring.
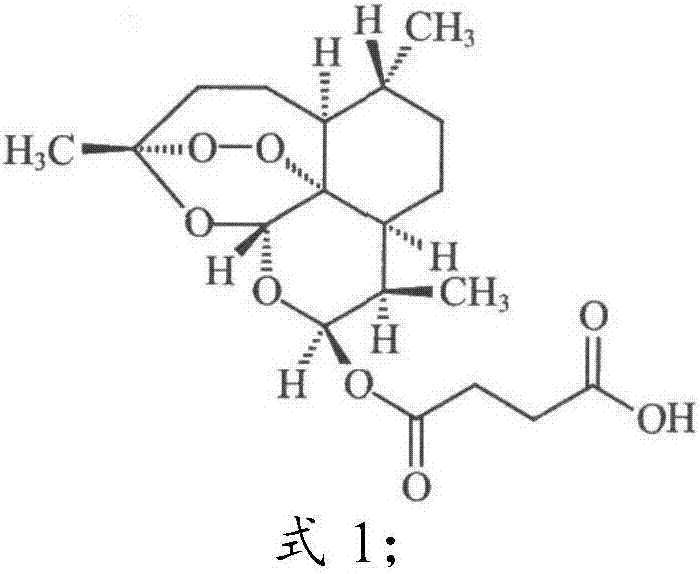
[0044] Spin-dry methanol under reduced pressure, add 100ml of 1N HCL, 300ml of water, 1000ml of EA for extraction, wash the organic phase with 500ml of 13% sodium chloride solution, wash with 500ml of saturated sodium chloride, dry the organic phase with anhydrous sodium sulfate for 1h, filter, reduce Press dry. After passing through a 200-300 mesh silica gel column, EA was eluted to obtain 40 g of sample ART2-Lys-OH, with a yield of 45% and a purity of 98.5% (HPLC).
Embodiment 3
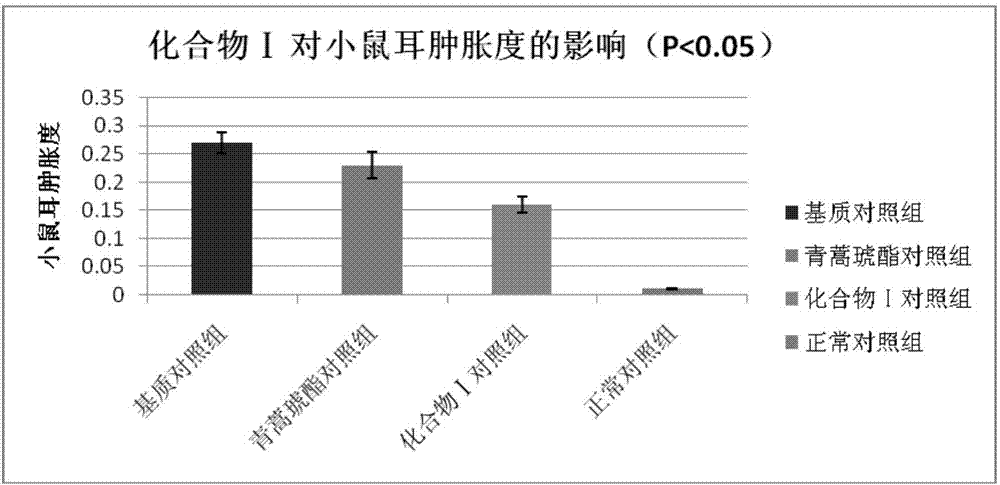
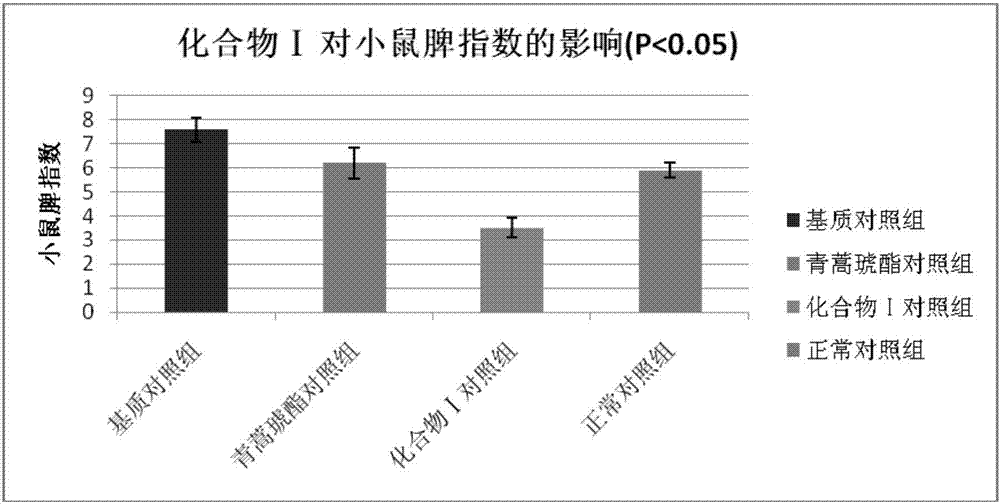
[0045]Embodiment 3 The compound of the present invention inhibits delayed hypersensitivity in mice
[0046] In the present invention, the effect of the compound ART2-Lys-OH prepared in Example 1 on the biological function of the ear and spleen of the delayed-type hypersensitivity mouse is discussed, and the immune regulation mechanism of compound I is explored, using the typical cellular immune response-delayed-type hypersensitivity The response (delayed-type hypersensitivity, DTH) was used as an index, and the immunomodulatory function of compound Ⅰ was explored by in vivo administration. DTH mice were established by referring to the literature (Hirasaki Y, Iwamura C, Yamashita M, et al. Repressor of GATA Negatively regulates murine contact hypersensitivity through the inhibition of type-2 allergic responses. Clin Immunol. 2011; 139(3): 267-276.) Model: 1 day before the experiment, the abdomen of the mice was shaved about 3 cm. On the first day (Day 0) and the first day, 40 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com