Method for controlling reaction temperature in preparation of dimethyl ether from methanol
A technology of reaction temperature and control method, which is applied in the dehydration of hydroxyl-containing compounds to prepare ethers, ether preparations, organic chemistry, etc. It can solve the problems of large temperature difference in the catalyst bed, short catalyst life, and easy aging of the catalyst, and achieve the start-up and operation process. The effect of simplicity, prolonging catalyst life, and ease of controlling and regulating the reaction zone temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
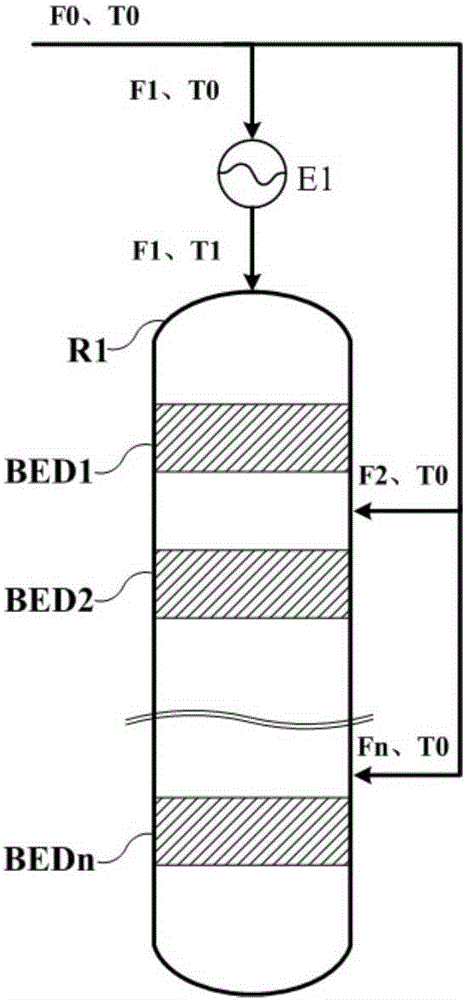
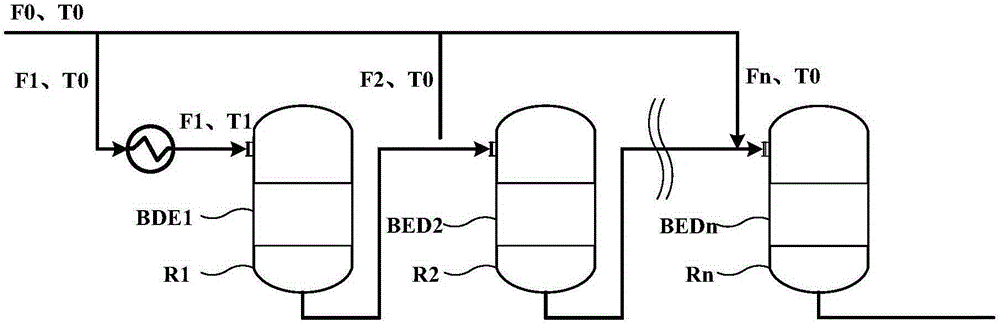
[0032] Based on 1000kg / h pure methanol feed, n is 6, according to Figure 1a Or the scheme shown in 1b is reacted, and the feed rate of the feed port of the first reaction zone is 600kg / h respectively, that is, K1=0.6, then according to the formula 50(1-K1)+245≤T1≤50(1-K1)+ 285 calculation can get 265≤T1≤305, T1 is 265, 285, 305 respectively. Pick Then the results of outlet temperature point temperature, hot spot temperature and methanol conversion rate of each reaction zone are shown in Table 1:
[0033] Table 1
[0034] a section of exit Second exit Three exits Fourth exit Fifth exit Six exits hot spot temperature methanol conversion T1=265 311 294 304 317 328 336 336 83.8% T1=285 330 307 316 328 339 347 347 83.9% T1=305 349 321 328 339 350 358 358 84.0%
Embodiment 2
[0036] Based on 1000kg / h pure methanol feed, n is 6, according to Figure 1a Or the scheme shown in 1b is reacted, and the feed rate of the feed port of the first reaction zone is 800kg / h respectively, that is, K1=0.8, then according to the formula 50(1-K1)+245≤T1≤50(1-K1)+ 285 calculation can get 255≤T1≤295, T1 is 255, 275, 295 respectively. Then the results of outlet temperature point temperature, hot spot temperature and methanol conversion rate of each reaction zone are shown in Table 2:
[0037] Table 2
[0038] a section of exit Second exit Three exits Fourth exit Fifth exit Six exits hot spot temperature methanol conversion T1=255 302 307 325 341 352 358 358 85.3%
[0039] T1=275 321 322 340 355 366 374 374 85.4% T1=295 340 338 355 369 380 380 380 85.5%
Embodiment 3
[0041] Based on 1000kg / h pure methanol feed, n is taken as 4, according to Figure 1a Or the scheme shown in 1b is reacted, and the feeding amount of the feed port of the first reaction zone is respectively 700kg / h, that is, K1=0.7, then according to the formula 50(1-K1)+245≤T1≤50(1-K1)+ 285 calculation can get 260≤T1≤300, T1 is 260, 280, 300 respectively. Then the results of outlet temperature point temperature, hot spot temperature and methanol conversion rate of each reaction zone are shown in Table 3:
[0042] table 3
[0043] a section of exit Second exit Three exits Fourth exit hot spot temperature methanol conversion T1=260 325 331 341 344 344 82.3% T1=280 343 346 355 356 356 82.6% T1=300 362 361 358 369 369 83.2%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com