Fluorescent probe with charge transfer properties and preparation method and use thereof
A fluorescent probe, charge transfer technology, applied in fluorescence/phosphorescence, chemical instruments and methods, organic chemistry, etc., can solve problems such as the need to improve the selectivity of action, and achieve low preparation cost, large market competitiveness, and probe structure. stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Synthesis of Fluorescent Probes
[0040] Dissolve 0.27 g of 4-formyltriphenylamine in 20 mL of n-butanol, add 0.38 g of 4-chloro-1,2-dimethylquinoline iodonium and 0.11 g of N,N-dimethylethyl Diamine, reacted at 120°C for 15h. After the reaction, it was naturally cooled to room temperature, and the solvent was distilled off. Add 50 mL of dichloromethane and stir for 10 minutes, filter to obtain a solid product, and then wash the solid twice with 20 mL of dichloromethane to obtain 0.21 g of pure product. The yield was 34.4%. H NMR 1 H NMR (400MHz, DMSO-d 6 )δ: 9.45(s, 1H), 8.86(s, 1H), 8.49(d, J=8.12Hz, 1H), 8.26(d, J=8.84Hz, 1H), 8.07(t, J=7.52Hz, 1H), 7.84-7.78(m, 4H), 7.56(d, J=15.76Hz, 1H), 7.40-7.36(m, 4H), 7.17-7.10(m, 6H), 6.99(d, J=8.12Hz ,2H),4.16(s,3H),4.03(s,2H),3.46(s,2H),2.90(s,6H). C NMR 1 C NMR (100MHz, DMSO-d 6 )δ:155.45,154.19,149.84,146.91,142.77,139.96,134.56,130.54,130.43,128.90,126.85,125.74,124.92,121.56,119.57,118.32,118.08,97.98...
Embodiment 2
[0041] Embodiment two: the stability of fluorescent probe
[0042] 1. The fluorescent probe was prepared into a 5 mM stock solution with DMSO solvent, and then diluted with a 10 mM Tris-HCl buffer solution (pH 7.4, containing 60 mM KCl) to a 10 μM probe solution for testing.
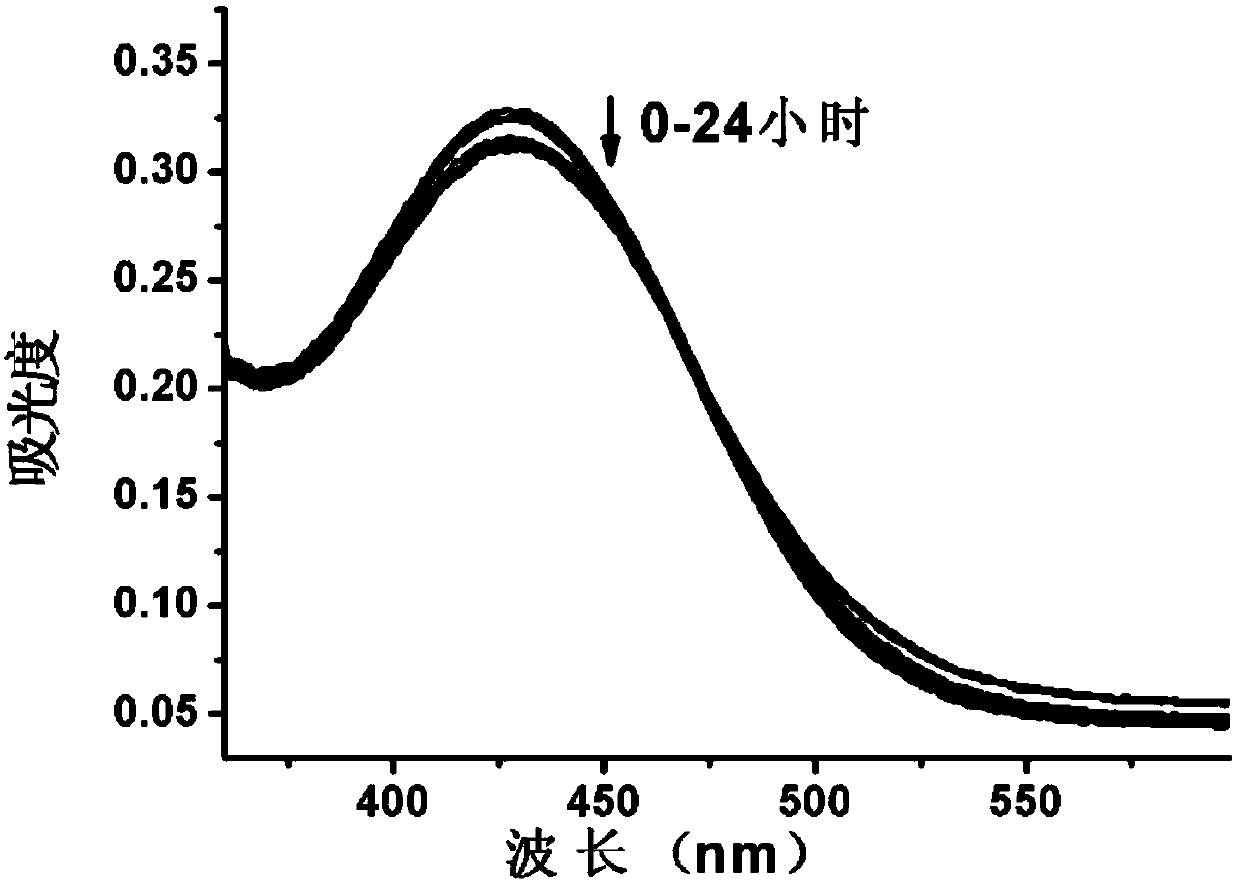
[0043] 2. Determination of ultraviolet absorption spectrum. Test the absorption spectrum of the above-mentioned probe solution at 360-600nm at the time of 0, 0.1, 0.5, 1, 2, 5, 7, 11, 20, and 24 hours with a UV spectrophotometer, so as to determine the fluorescence of the fluorescent probe. stability. The result is as figure 1 As shown, the ultraviolet absorption of the probe did not change significantly within 0-24 hours, and no new absorption peak appeared, indicating that the probe could stably exist in the solution under the experimental conditions.
Embodiment 3
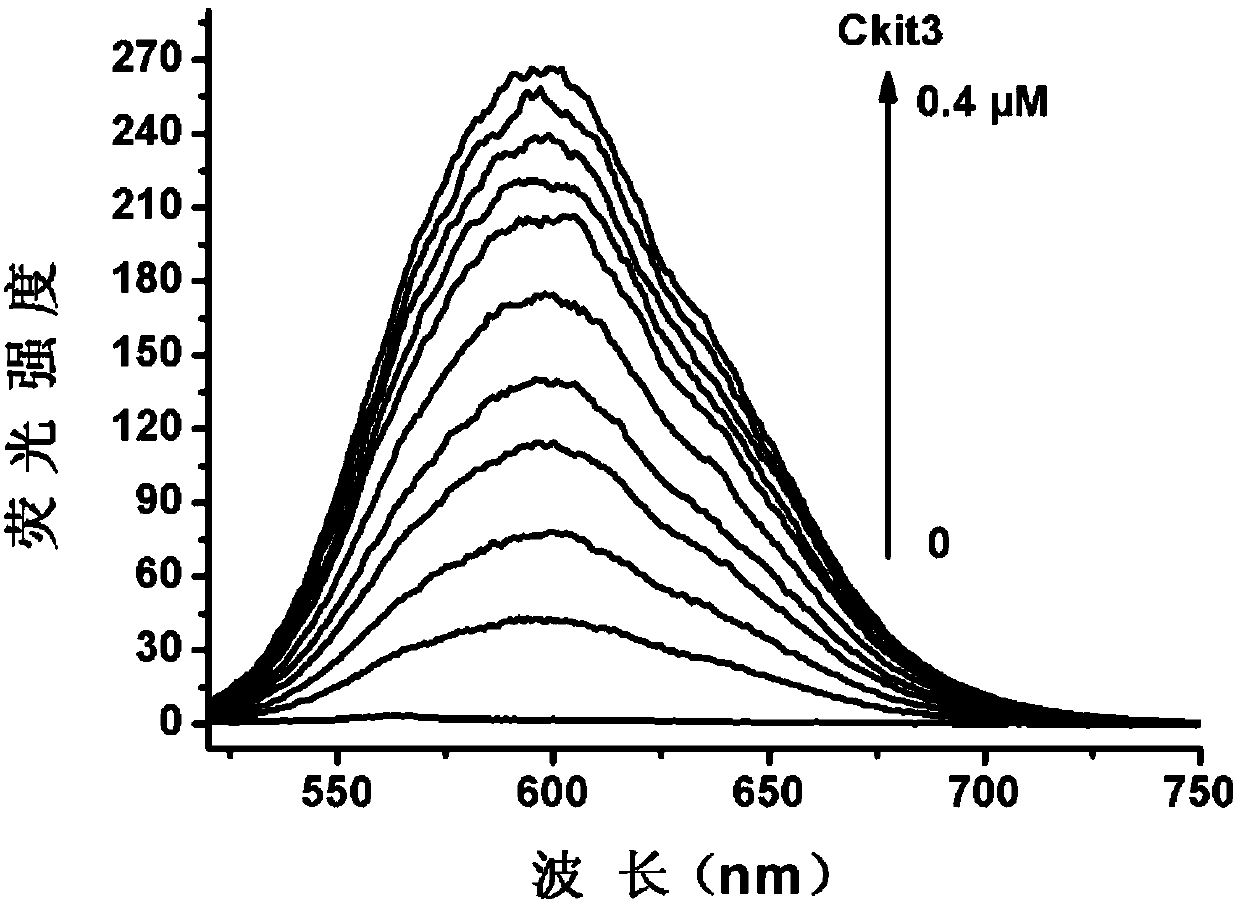
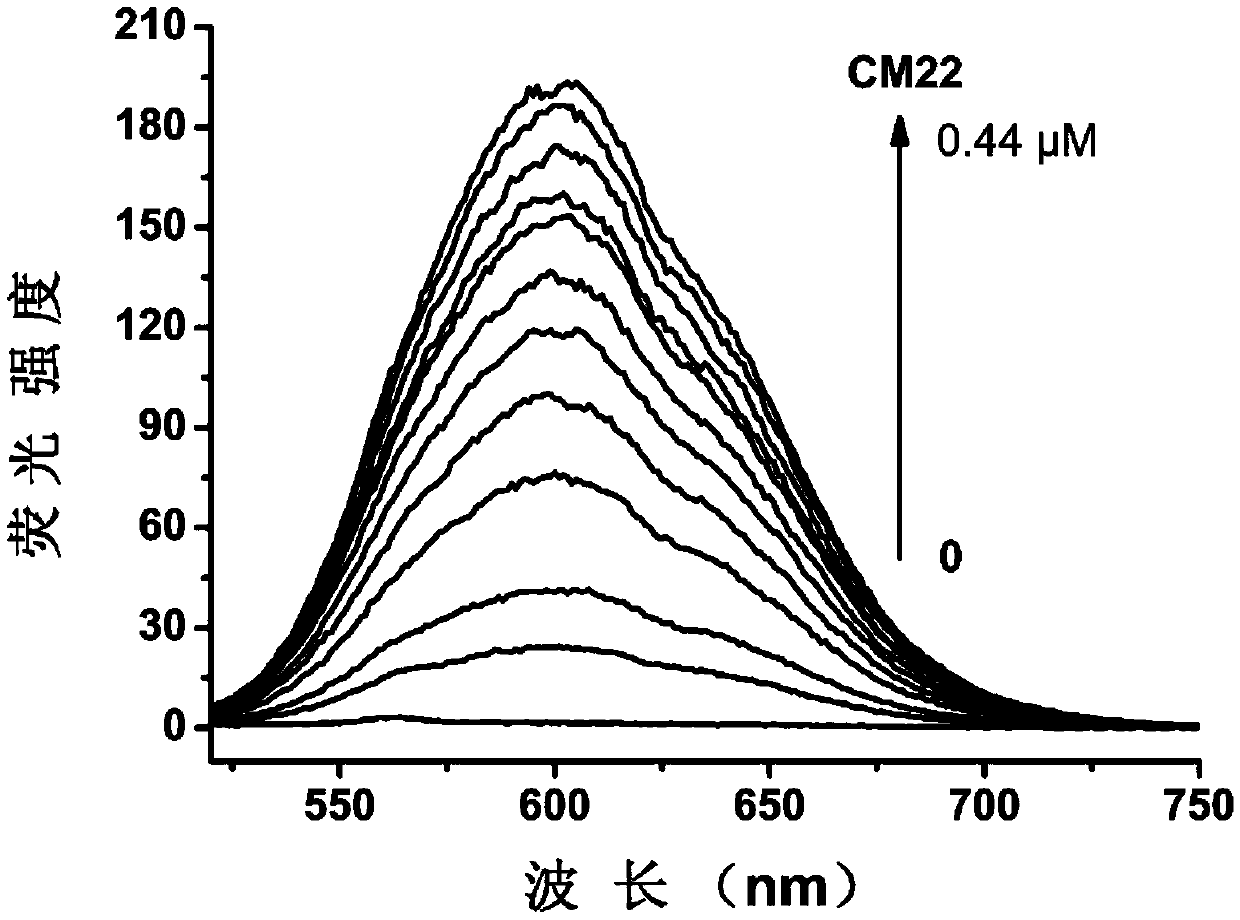
[0044] Embodiment three: the detection of DNA sample
[0045] 1. DNA sample preparation. DNA samples were purchased from Shanghai Sangon Co., Ltd. The DNA sample was dissolved in an appropriate amount of buffer solution (10mM Tris-HCl, pH 7.4, 60mM KCl), and its concentration was calculated according to the ultraviolet absorption value and molar absorptivity of the sample solution at 260nm.
[0046] Wherein, the DNA sequence used is:
[0047] Ckit3: GGCGAGGAGGGGCGTGGCCGGC
[0048] CM22: TGAGGGTGGGTAGGGTGGGTAA
[0049] HRAS: TCGGGTTGCGGGCGCAGGGCACGGGCG
[0050] c-myc: TTGAGGGTGGGTAGGGTGGGTAAA
[0051] 22AG: AGGGTTAGGGTTAGGGTTAGGG
[0052] G3T3: GGGTTTGGGTTTGGGTTTGGG
[0053] htg-21: GGGTTAGGGTTAGGGTTAGGG
[0054] Ckit1: AGGGAGGGCGCTGGGAGGAGGG
[0055] ds26: CAATCGGATCGAATTCGATCCGATTG
[0056] polyd(A-T) 9 : ATATATATATATATATAT
[0057] polyd (G-C) 9 : GCGCGCGCGCGCGCGCGC
[0058] ct-DNA: calf thymus DNA
[0059] ss26: CCGCGAACGCCTAAGCTGCTAACCGC
[0060] 2. Prepara...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com