Application of compound hu-17 alone or in combination with tyrosine kinase inhibitors in the preparation of drugs for the treatment of chronic myelogenous leukemia
A chronic granulocyte and tyrosine kinase technology, which is applied in the field of medicine and biology, can solve the problems of unsatisfactory curative effect, slow release rate of less than 40%, and poor curative effect of patients in blast stage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1. Preparation of compound Hu-17
[0021] The chemical structural formula of compound Hu-17 is as follows:
[0022]
[0023] Molecular formula: C 63 h 96 N 4 o 11 , Molecular weight: 1084Da.
[0024] Phytolaccagenin (phytolaccagenin) is a kind of active ingredient separated from the traditional Chinese medicine Pokweed, and it is the aglycone part of Phytolacca saponin A (Esculentoside A, EsA), and Phytolaccagenin in the present invention is obtained through acid hydrolysis. Obtained from saponin A, its structure is as follows:
[0025]
[0026] Dissolve pokeweed saponin (26.6mg, 0.05mmol) in dry 2ml DMF / THF (1:3, v / v), add N,N-dicyclohexylcarboimide (DCC, 20.7mg, 0.1 mmol) and N-hydroxybenzotriazole (HOBt, 13.5 mg, 0.1 mmol), stirred at room temperature for 3 hours. After the completion of the reaction, concentrate under reduced pressure to dryness, dissolve the residue with dichloromethane (20ml), filter, and concentrate the filtrate to dryness...
Embodiment 2
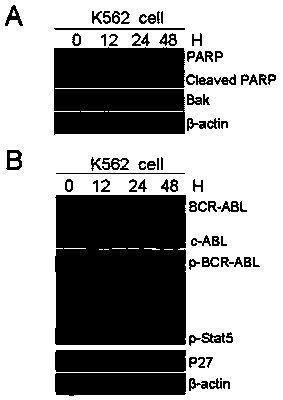
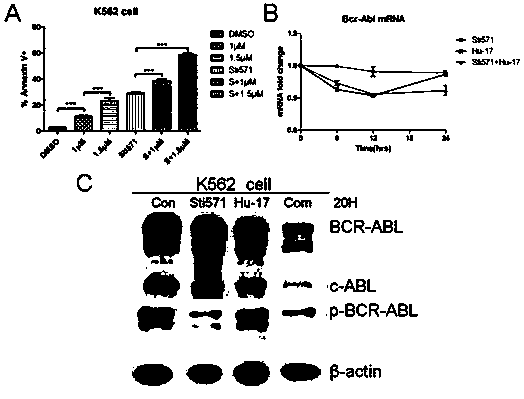
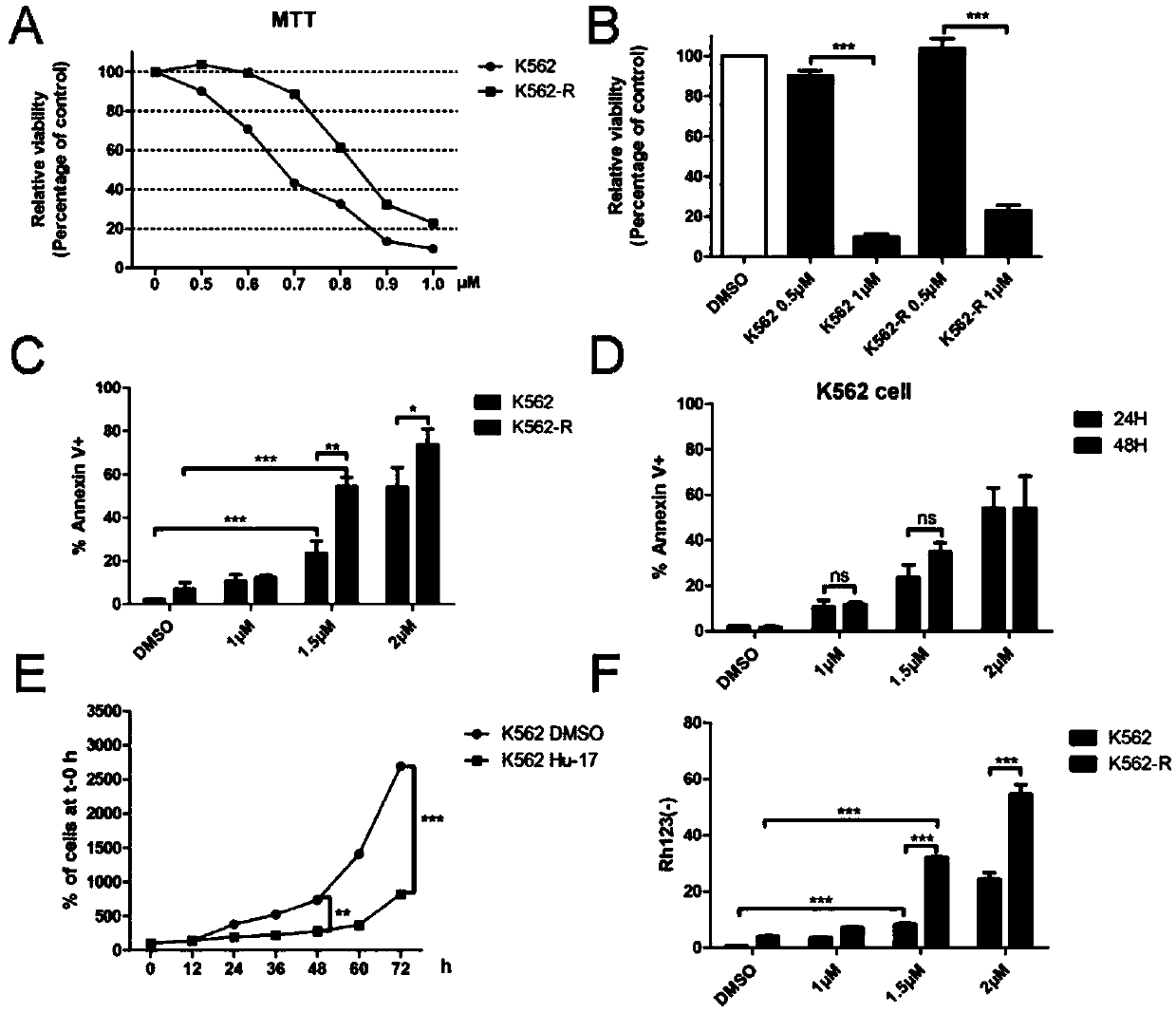
[0027] Example 2. Application of compound Hu-17 alone or in combination with tyrosine kinase inhibitors in the preparation of drugs for the treatment of chronic myeloid leukemia
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap